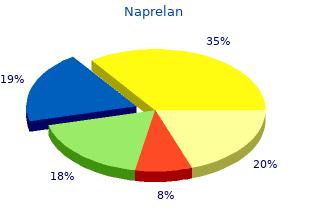
Naprelan
"Naprelan 250mg line, pain treatment scoliosis."
By: Bertram G. Katzung MD, PhD
- Professor Emeritus, Department of Cellular & Molecular Pharmacology, University of California, San Francisco

http://cmp.ucsf.edu/faculty/bertram-katzung
In men order naprelan 250 mg without a prescription pain treatment center of the bluegrass lexington ky, the infectious agent invades the prostate order naprelan 500 mg overnight delivery pain medication for dogs rimadyl, urethra or seminal vesicles; it often causes only mild symptoms but may cause as much as 5%?10% of nongonococcal urethritis in some areas 250 mg naprelan with mastercard pain home treatment. Occurrence?Widespread; a frequent disease naprelan 500mg amex pain treatment ulcerative colitis, primarily of adults, with the highest incidence among females 16?35 years. Overall, about 20% of females may become infected during their reproductive years. Mode of transmission?Through contact with vaginal and urethral discharges of infected people during sexual intercourse. Incubation period?4?20 days, average 7 days; many are symptom-free carriers for years. Period of communicability?For the duration of the persistent infection, which may last years. Susceptibility?Susceptibility to infection is general, but clinical disease is seen mainly in females. Preventive measures: Educate the public to seek medical advice whenever there is an abnormal discharge from the genitalia and to refrain from sexual intercourse until investigation and treatment of self and partner(s) are completed. Promotion of safer sex behaviour, including condom use, is recommended for all sexual contacts where mutual monogamy is not the case. Cases of metronidazole resistance have been reported and should be treated with topical intravaginal paromomycin. Diagnosis is made through demonstration of eggs in feces or sigmoidoscopic observation of worms attached to the wall of the lower colon in heavy infections. Infectious agent?Trichuris trichiura (Trichocephalus trichiurus) or human whipworm, a nematode. Mode of transmission?Indirect, particularly through pica or ingestion of contaminated vegetables; no immediate person-to-person transmission. Eggs passed in feces require a minimum of 10?14 days in warm moist soil to become infective. Hatching of larvae follows ingestion of infective eggs from contaminated soil, attachment to the mucosa of the caecum and proximal colon, and development into mature worms. Eggs appear in the feces 70?90 days after ingestion of embryonated eggs; symptoms may appear much earlier. Preventive measures: 1) Educate all members of the family, particularly children, in the use of toilet facilities. In the early stage, a painful chancre, originating as a papule and evolving into a nodule, may be found at the primary tsetse? Parasite-concentration techniques (capillary tube centrifugation, or minianion exchange centrifugation) are almost always required in gambiense and less often in rhodesiense disease. Inoculation on laboratory rats or mice is sometimes useful in rhodesiense disease. Standard bioclinical parameters such as anemia and thrombocytopenia may provide indirect diagnostic evidence for trypanosomiasis. Wild animals, especially bushbucks and antelopes, and domestic cattle are the chief animal reservoirs for T. Direct mechanical transmission by blood on the proboscis of Glossina and other biting insects, such as horse? Parasitemia in humans occurs in waves of varying intensity in untreated cases and occurs at all stages of the disease. Occasional inapparent or asymptomatic infections have been documented with both T. Preventive measures: Selection of appropriate prevention methods must be based on knowledge of the local ecology of vectors and infectious agents. In a given geographic area, priority must be given to one or more of the following: 1) Educate the public on personal protective measures against tsetse? Control of patient, contacts and the immediate environment: 1) Systematic screening of exposed populations in each T. Early diagnosis reduces both the risk of sequelae and the drug-related risks, and helps stop transmission. Regular surveillance in local health centers and villages for both rhodesiense and gambiense areas. Report to local health authority: In selected endemic areas, establish records of prevalence and encourage control measures; not a reportable disease in most countries, Class 3 (see Reporting). Treatment of the neurological phase requires a drug that can cross the blood-brain barrier. Early diagnosis, allowing low-risk treatment on an outpatient basis, should be attempted in remote rural settings where the disease takes its heaviest toll. While some are well tolerated, in others?used in the neurological phase?fatal complications are common. Problems of drug resistance have increasingly been reported in several countries. The treatment of sleeping sickness depends on 5 key drugs needed for the different forms and stages of the disease. This drug must be administered in hospital and if possible in the intensive care unit. Patients treated must be re-examined for at least one and preferably 2 years for possible relapses C. If epidemics recur despite initial control measures, the measures recommended in 9A must be pursued more vigorously. In 20%?30% of infections, irreversible chronic manifestations generally appear later in life. Unilateral bipalpebral-oedema (Romana sign) occurs in a small percentage of acute cases. Life-threatening or fatal manifestations include myocarditis and meningoencephalitis.
Explanation: Due to generic naprelan 250 mg with visa pain treatment in hindi the potentially serious toxicity associated with preparative regimens cheap naprelan 500mg amex pain treatment in sickle cell, Clinical Programs must verify that cellular therapy products or suitable donors will be available prior to buy 250 mg naprelan mastercard ohio valley pain treatment center administering preparative regimens cheap naprelan 250mg on line joint & pain treatment center. There are risks involved in the distribution of cellular therapy products, such as damage to the product container and significant warming events. Ordinarily, cryopreserved cellular therapy products should be chosen, ordered, and transported and/or shipped early enough in the process that the unit(s) will be on-site prior to the start of the preparative regimen. In the event there are problems encountered during transport and/or shipping or discovered upon arrival of the product, the recipient will not be at risk. Cellular therapy products should be assessed to confirm quality and adequacy of dose. Alternatively, Clinical Programs may include a description of a process evident in dictated notes. Explanation: Cellular therapy products obtained from registries or manufacturers outside of the cellular therapy program may differ in important ways for which the Processing Facility must be prepared. Required preparations may include special storage arrangements, necessary supplies and reagents, and developing personnel competency in order to process the product for administration while protecting cell viability and product safety. Explanation: Preparative regimens encompass various modalities, such as biologic, radiologic, and chemotherapy. It is recommended that a tracking system regarding mixture, delivery, and completed administration be instituted for all these regimens. Staff administering the preparative regimen shall be appropriately credentialed as defined by institutional policies and in accordance with governmental laws and regulations. Example(s): Administration of chemotherapy in the preparative regimen context requires specific policy(ies) for safe administration due to the risk of adverse outcomes related to high doses. One formulation must be reconstituted and infused within a 60-minute period; a newer formulation remains stable for five hours (or more if refrigerated). If a Clinical Program begins collaboration on immune effector cell programs with hematologists/oncologists not experienced with cellular therapy, some explanation of the preparative regimen will be necessary. Explanation: It is recognized that treatment orders must be approved by various individuals; however, the height and current weight should be measured and recorded before treatment administration. Explanation: A protocol or standard of care-specific set of orders that are preprinted and readily available in written or electronic form is an important measure of control; however, it is still critical that the drug doses are verified and documented by an attending physician prior to transmitting the order to the pharmacy. A final checklist is required to confirm each step in preparing for and administering therapy is performed prior to cellular therapy product administration. Written instructions should be available for reconstitution, dilution, mixing, labeling, and packaging. There should be a standard process in place to retrieve the batch number and expiry of all drugs and diluents used in the preparation of the therapy regimens. Evidence: Copies of standard treatment or research protocols in areas of recipient care such as inpatient and outpatient units and the pharmacy can provide evidence of compliance. Specific patient charts can be used to check that treatment orders and documentation are compliant with the guidelines. While touring patient care areas, the inspector may also ask the pharmacists about their normal practice and if they retain ultimate responsibility for verification against the protocol or standard regimen listed on the orders. Nurses may be asked about the normal procedures for treatment administration to confirm this. Explanation: In writing as used in the Standards includes electronic documentation. Information from the radiation oncology consultation, including factors that may increase the toxicity of the radiation, should be discussed with the patient and informed consent should be documented. Documentation that the radiation was given on a specific date and its dose can be compared to the consultation documentation. The inspector can also ask to see copies of treatment protocols that include radiation and verify the protocol by comparing it to patient charts. Explanation: Non-cryopreserved (often referred to as fresh?) cellular therapy products must be administered within the time specified by Clinical Program policies, registry and tissue bank requirements, and applicable laws and regulations. It may be optimal to thaw individual bags to reduce the time thawed products sit before administration. Clinical Programs must identify appropriate timeframes between the end of the preparative regimen and administration of the cellular therapy product to confirm that the administered product is not affected by the preparative regimen. The program must verify that the preparative regimens were given at the scheduled time and delay administration of the cells if required. Programs are responsible for communicating with the Processing Facility regarding any delayed administration. Clinical Programs need to determine the composition of the cellular therapy product to determine how it should be prepared for administration. Programs should work with their Processing Facilities to verify appropriate processing and preparation of the product for administration. Evidence: Staff should be prepared to discuss their normal practice and their training in the administration of cellular therapy products. Specific patient charts can be used to determine that two persons checked the product and that the documentation in the chart is complete. If there is time and an administration is scheduled on the day of inspection, the inspector should be notified so that he/she may watch parts of the procedure. One way Clinical Programs can communicate date and time of administration to the Processing Facility is to use a facesheet or other written documentation of the start and end date of the preparative regimen and the date and time, if needed, of the cellular therapy product administration. If plans change, updated information is provided to the laboratory prior to the planned day of administration.
The authors commented on the aforementioned differing laser-tissue interactions of thulium and holmium order 500 mg naprelan unifour pain treatment center statesville nc. Mean operating time was 100 minutes (range generic naprelan 500mg free shipping low back pain treatment kerala, 40?220 min) and operating efficiency was 0 generic naprelan 250 mg fast delivery gosy pain treatment center. There was one ureteric orifice injury managed by stent Surgical Therapies and New Treatments 265 placement naprelan 250 mg without prescription heel pain treatment youtube. One patient was transfused, and 13% of patients had delayed hematuria which did not require surgical intervention. It is not possible to interpret the complication rates given the small number of patients. Although thulium can be used for vaporization, resection, and enucleation, resection is the most studied thulium technique. Its use for vaporization and enucleation should be limited to the context of randomized clinical trials for the time being until more, good-quality evidence is available. The structure of the semiconductor bar can be varied by using different elements and layer structures in varying combinations. Each of these wavelengths can have very different interactions with tissues, and the diode lasers are grouped together solely on the basis of their shared method of generation. There were no significant differences in baseline characteristics and peri-operative data except for mean applied energy, which was higher for diode (318 kJ) than for GreenLight (206. Mean prostate volume, laser time, catheterization time, and hospital time for diode versus GreenLight were 66. Mean reduction in prostate volume at 6 months was 52% for diode and 38% for GreenLight. In terms of complications, 12% of GreenLight cases needed electrocautery to control bleeding compared with none in the diode group. The GreenLight laser is highly absorbed by Hb and when it encounters a large vessel it induces vapour bubbles within it, which can tear the vessel wall. Transient re-catheterization was required in 12% in the GreenLight group compared with 11% in the diode group. There were significant differences favouring GreenLight in terms of transient incontinence (14. The deeper coagulation zone induced by the diode laser might account for these differences. Severe intra-operative bleeding that impaired vision was noted in 13% of GreenLight cases and none of the diode cases. Only 4% of diode cases needed post-operative irrigation compared with 40% of GreenLight cases. Capsular perforation occurred in 5% of GreenLight cases and caused severe bleeding in one patient who required a blood transfusion. Some complications were more common in the diode group including transient urge incontinence (7% vs. Most confirm the excellent hemostatic and vaporizing properties of the diode laser, but some raise concerns over deeptissue damage that results in a number of complications including tissue sloughing and dysuria (183?187). Modification of the fibre used for diode vaporization has led to some reduction in complications and side effects (188). Modification of the wavelength and/or pulse mode may also be necessary to reduce the depth of tissue damage. Samples ranged in size from 4 mm to 30 mm and had brownish margins with a coagulation rim of 0. The evidence for diode resection and enucleation is too limited currently to make any firm recommendation other than that more good-quality clinical trials are required. Surgical Therapies and New Treatments 269 Minimally invasive surgical treatments usually cover procedures that do not lead to the physical removal of prostatic tissue. Laser prostate treatments are not included in this section, as they are generally used to remove tissue by ablation, resection, or enucleation. Relevant full-text papers were obtained and additional references found in papers and books reference lists. The alternating current is at radio frequency, and the effects on tissue are the creation of lesions leading to coagulative necrosis. A long needle with two electrodes at its tip is used to puncture the prostate endoscopically under direct vision (Figure 3). Typically, one puncture and treatment is made for each 5-g increment in prostate size. While initial outcomes by 12 months were meaningful, only one patient had continued symptom benefit at 5 years. Few adverse events were reported, with the only one of significance being a death within 24 hours of the procedure due to cardiovascular disease. With the majority of men in this study having short followup, it is likely that the true treatment failure rate in terms of re-operation or continued catheterization is higher than reported. There have, however, been publications on imaging and others dealing with adverse events, particularly urinary retention. As this is an update from a similar review by the same authors and in the same journal in 2007 (197), only the findings from the 2012 study will be considered. The most recent of these studies that was considered to have provided evaluable effectiveness data had been published in 2002. Five studies have since reported longer-term results and are summarized in Table 11. One of these studies (198) was focused on demonstrating that smaller prostates had as good outcomes as largersized prostates in terms of symptom scores and flow rates. Only 156 men had evaluable symptoms score data and 46 had flow data at 5 years of follow-up.

Demetrios M order 250 mg naprelan visa lower back pain treatment exercise, Khan F cheap naprelan 250mg line pain treatment consultants of wny, Turner-Stokes L purchase naprelan 250 mg without a prescription midsouth pain treatment center reviews, Brand C and McSweeney S (2013) Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity discount naprelan 250 mg on-line tennova comprehensive pain treatment center. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 91(9):729?46. French B, Thomas L, Leathley M et al (2010) Does repetitive task training improve functional activity after stroke? Gericke T (2006) Postural management for children with cerebral palsy: consensus statement. Glinsky J, Harvey L and Van Es P (2007) Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: a systematic review. Greene P and Fahn S (1992) Dvelopment of antibodies to botulinum toxin type A in torticollis patients treated with botulinum toxin injections. Greene P and Fahn S (1993) Development of antibodies to botulinum toxin type A in patients with torticollis treated with injection of botulinum toxin type A. Hambleton P, Pickett A and Shone C (2007) Botulinum toxin: from menace to medicine. Hatherway C and Deng C (1994) Immunogenicity of the neurotoxins of Clostridium botulinum. Hesse S, Reiter F, Konrad M and Jahnke M T (1998) Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Hyman N, Barnes M, Bhakta B et al (2000) Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study [see comment]. Jackson D, Horn S, Kersten P and Turner-Stokes L (2006) Development of a pictorial scale of pain intensity for patients with communication impairments: initial validation in a general population. Jankovic J and Schwartz K (1995) Response and immunoresistance to botulinum toxin injections. Jolk C, Alcantara R, Bernhardt L, Platen P, Marziniak M and Wessling K (2012) Effects of 24 weeks progressive resistance training in comparison to core and stability training performed in groups on muscle performance, balance and spasticity in people with multiple sclerosis. Kaji R, Osako Y, Suyama K, Maeda T, Uechi Y and Iwasaki M (2010) Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. Keenan M, Haider T and Stone L (1990) Dynamic electromyography to assess elbow spasticity. MacFarlane A, Turner-Stokes L and De Souza L (2002) the associated reaction rating scale: a clinical tool to measure associated reactions in the hemiplegic upper limb. Mehrholz J, Wagner K, Meissner D et al (2005) Reliability of the modified Tardieu scale and the modified Ashworth scale in adult patients with severe brain injury: a comparison study. Moore A and Naumann M (2003) General and clinical aspects of treatment with botulinum toxin. National Institute of Health and Care Excellence (2013a) Patient Group Directions. National Institute of Health and Care Excellence (2013b) Stroke rehabilitation in adults. National Institute of Health and Care Excellence (2014) Multiple sclerosis in adults: management. Novotna A, Mares J, Ratcliffe S, Novakova I et al Sativex Spasticity Study Group (2011) A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex?), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Nursing and Midwifery Council (2006) Standards of proficiency for nurse and midwife prescribers. O?Dell M, Brashear A, Jech R et al (2017) Dose-dependent effects of AbobotulinumtoxinA (Dysport) on spasticity and active movements in adults with upper limb spasticity: secondary analysis of a phase 3 study. Reiter F, Danni M, Lagalla G, Ceravolo G and Provinciali L (1998) Low-dose botulinum toxin with ankle taping for the treatment of spastic equinovarus foot after stroke. Richardson D, Greenwood R, Sheean G, Thompson A and Edwards S (2000) Treatment of focal spasticity with botulinum toxin: effect on the positive support reaction. Royal College of Physicians, British Society of Rehabilitation Medicine, Chartered Society of Physiotherapy and Association of Chartered Physiotherapists Interested in Neurology (2009) Spasticity in adults: management using botulinum toxin. Effect of botulinum toxin type A, motor imagery and motor observation on motor function of hemiparetic upper limb after stroke. Schiavo G, Benfenati F, Poulain B et al (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Simpson D, Gracies J, Yablon S, Barbano R and Brashear A (2009) Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. Simpson D, Hallett M, Ashman E et al (2016) Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Sun S, Hsu C, Sun H, Hwang C, Yang C and Wang J (2010) Combined botulinum toxin type A with modified constraintinduced movement therapy for chronic stroke patients with upper extremity spasticity: a randomized controlled study. Teplicky R, Russell D and Law M (2003) Casts, splints, and orthoses Upper extremity review of effectiveness literature for children with neurological disorders. Turner-Stokes L and Ashford S (2007) Serial injection of botulinum toxin for muscle imbalance due to regional spasticity in the upper limb. Turner-Stokes L, Baguley I, De Graaff S, Katrak P, Davies L, McCrory P and Hughes A (2010) Goal attainment scaling in the evaluation of treatment of upper limb spasticity with botulinum toxin: A secondary analysis from a double-blind placebo-controlled randomised clinical trial. The management of adults with spasticity using botulinum toxin: a guide to clinical practice. These smaller organisations typically lack the time, resources and machinery for the full systematic approach adopted by formal guidelines.
A high percentage of animals is slaughtered for human consumption at an age when typical lesions have not yet become detectable (except in those countries where leukoencephalomyeUtis occurs in kids aged 1 to order naprelan 500 mg free shipping midwest pain treatment center fremont ohio 4 months old) (7) cheap 250mg naprelan free shipping pain medication for dogs post surgery. The classical form of the disease consists of arthritis buy 250mg naprelan with mastercard pain treatment and wellness center greensburg pa, which may or may not be complicated by the presence of bursitis and synovitis buy naprelan 500 mg with amex best pain medication for a uti. The changes occur predominantly in the carpal joints, although other joints may be involved. The leukoencephalomyeUtis observed in young goats, as weU as the granulomatous encephalomyeutis seen in older goats, are not as widely distributed as is arthritis. This is because this aspect of the disease has not yet been reproduced experimentaUy. If an animal shows the characteristic signs of arthritis, with or without the presence of bursitis, which predominantly affects the carpal joints, material should be aspirated by syringe from the affected joint cavities. If it is possible to sacrifice the animal, smaU samples of the cartilage or the synovial membrane of the affected joints, together with synovial cells, can be used to establish tissue culture expiants and to inoculate into synovial tissue or cartilage ceU culture monolayers. Cultures of other ceU types can also be employed, such as those of the choroid plexus, fetal testis or from the cornea. In tests on culture media that are carried out for the detection of virus, the fluids should first be concentrated at least 100-fold. If other tests are conducted on culture media subsequently, any of the available serological tests can be appued. Details for this procedure may be found in the chapter on maedi-visna (B33, Chapter 47). When the disease is endemic, the investigation of new outbreaks should start with the demonstration of antibodies either in sera or in milk, preferably colostrum. In most cases, however, these will be sufficient, particularly as it is still uncertain whether viruses of the maedi-visna group occur in goats. Identification of the agent: Differentiation of the above-mentioned aetiological agents depends on the use of suitable media for isolation and on carrying out biochemical and serological tests on the isolates. Requirements for biological products: Killed vaccines are not considered efficacious for prevention of M. Live vaccines made from attenuated cultures have shown better results than dead vaccines with M. It occurs mainly in Europe, Western Asia and North Africa, and is caused by Mycoplasma agalactiae (4, 6). The clinical signs in these infections were sufficiently similar to those of contagious agalactia to lead Perreau (28) to suggest that these infections could also be considered as contagious agalactia. In addition, very recent cases of mastitis and arthritis in goats associated with M. Whether regulatory authorities will prefer to preserve the name for the disease caused by M. Bacteraemia is common and could account for the isolation of the organism from sites where it is only transiently present. Mastitis, arthritis, pleurisy, pneumonia and keratoconjunctivitis may all result from infection with M. Usually cases occur sporadically but the disease may smoulder and slowly spread within a herd. After parturition the opportunity for spread in does being milked increases and kids ingesting infected colostrum and milk become infected. The resulting septicaemia with arthritis and pneumonia gives rise to high mortality in the kids (9,11). Goats are more commonly affected than sheep and clinical signs of fever, septicaemia, mastitis and severe arthritis may be followed rapidly by death (6, 8). The severe joint lesions (8) seen in experimental infections with this organism are accompanied by intense periarticular subcutaneous oedema affecting tissues to some distance from the joint. Quite recently in California, a large outbreak of mastitis and agalactia in goats was apparently caused by a pure infection with M. Severe arthritis later became a prominent sign in does and kids although at no time was pyrexia seen in infected goats (10). Identification of the agents the usual techniques used in the isolation of mycoplasmas apply to all four organisms under discussion. Media containing heart infusion broth, yeast extract (1-2%), and horse or pig serum (15-20%), with bacterial inhibitors, ampicillin (150 mg/htre) and thallous acetate (250 mg/litre) will be adequate to isolate and grow M. However, thallous acetate has not been used in some countries because it inhibits the growth of certain mycoplasmas. Sites of choice for culture are, in the living animal, milk, blood, aspirated joint fluid and nasal exudate, and at necropsy, udder and associated lymph nodes, joint fluid and lung lesions. The organisms may be isolated from liver, kidney and spleen when bacteraemia is present. The solid medium is incubated in a moist atmosphere at 37?C in 5% C0 or in a2 candle jar (3% C02). Liquid medium is incubated at 37?C and plated on solid medium when growth is evident, or at 5-7 days even if growth cannot be seen. From well separated colonies single representatives are picked off into liquid medium and incubated. The colony appearance is useful only to the experienced eye, but selected biochemical tests (7,12) can help with initial screening.
Discount naprelan 250mg amex. Gallstones and Surgical Removal of Gallbladder (Cholecystectomy) Animation..