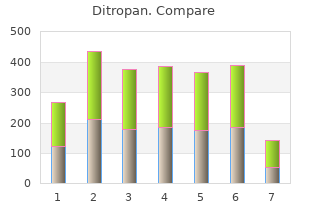
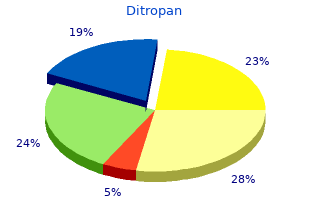
Ditropan
"Ditropan 5 mg sale, gastritis symptoms depression."
By: Bertram G. Katzung MD, PhD
- Professor Emeritus, Department of Cellular & Molecular Pharmacology, University of California, San Francisco

http://cmp.ucsf.edu/faculty/bertram-katzung
We also observed a significant overall decrease in 2016 among both vulnerable (76% in 2014 vs 65% in 2016; P=0 purchase ditropan 5mg with visa gastritis diet 123. A significant overall decline was observed regardless of social vulnerability status buy ditropan 2.5mg mastercard chronic gastritis yahoo answers. Disturbing significant decreases were observed among the younger age-group (women aged 50-54 yrs) of the target population of the organized program purchase ditropan 2.5mg with amex gastritis ulcer medicine, and among unemployed women cheap 2.5 mg ditropan fast delivery gastritis root word. Although organized programs have been shown to ensure equitable access to cancer screening, this achievement remains precarious and requires regular monitoring. With respect to local therapy, younger women also had a greater tendency to undergo bilateral mastectomy (<40 years, 34. Women at both age extremes (≥70 years and <40 years at diagnosis) demonstrate worse cancer-specific survival outcomes. In older women, this may be due to undertreatment, and in younger women, to delays in diagnosis and/or worse tumor biology. Anam Hospital, Korea University College of Medicine, Seoul, Korea; Anam Hospital, 3 Korea University College of Medicine, Seoul, Korea; Anam Hospital, Korea University College of Medicine, Seoul, Korea and 4 Anam Hospital, Korea University College of Medicine, Seoul, Korea. Although reported incidence from pivotal trials is very low and acceptable, no big data-based population study has not been conducted in Koreans yet. Interaction between age and tumor subtype was tested using a Cox regression model. Variation could exist depending on access to care, insurance availability, geographic area, and treatment options (urban vs. For the institutional cohort time to event used the outcome death from breast cancer confirmed from the patient chart or death certificate if information on cause of death was not available in the chart. Better survival in the Seattle-Puget Sound region is supported by the retrospective cohort analysis results from a center with a more detailed registry and complete follow up in the same region. In order to develop successful interventions we need to understand the major contributing factors. Osteopenia and osteoporosis were ascertained based on self-reported physician diagnosis in baseline and follow-up questionnaires. Future studies are needed to address the frequency of monitoring among specific age and treatment groups. Body: Background: Breast cancer is the most common cancer among women and a significant number of women experience recurrence. Statins, drugs for lowering cholesterol, were introduced in 1987, and by 2011-12, 27. Statins may impact other diseases, beyond cardiovascular disease, including cancer. Statin use and breast cancer recurrence or disease-free survival has previously been explored in 8 cohort studies and 1 case-control study with mixed results. The index date was the recurrence date for the case and the date for an equivalent period after diagnosis for the matched control. We collected data from medical records and from pharmacy, laboratory, tumor registry, and membership health plan databases. We performed bivariate analysis to look at characteristics associated with recurrence. A priori, we identified potential confounding variables based on literature review and clinical knowledge. Using multivariable logistic regression analysis, we assessed statin use in relation to breast cancer recurrence, accounting for factors that may alter the association. Results: We identified 306 cases with breast cancer recurrence and 679 matched controls. We calculated dose equivalents for all statins, using 20 mg of simvastatin as one dose. Among those who took statins, the average number of equivalent doses per day after diagnosis was 1. Conclusions: While other studies have reported that statins may be associated with decreased odds of breast cancer recurrence, our preliminary multivariable analyses that looked at any statin use between diagnosis and index date do not support those results. Phthalates interfere with hormonal signaling and may affect reproductive, developmental, and cancer endpoints. Associations between phthalates and breast cancer incidence have not been thoroughly investigated. Users of phthalate-containing medications have up to 70-fold higher urinary phthalate levels than other individuals, and represent a highly exposed population for efficient study of phthalate health effects. We used the Danish Drug Information Database to identify all phthalate-containing oral medications marketed during the study period. We identified a nationwide cohort of women at risk for a first cancer between 2005—2015, and who had no previous exposure to a phthalate-containing drug. Using the National Prescription Registry we characterized time-varying, medication-borne phthalate exposure. We fit Cox regression models to estimate associations between cumulative phthalate exposures and breast cancer incidence. Fourteen percent of the cohort (n=161,751) was prescribed a phthalate-containing drug. Key Laboratory of Breast Cancer in Shanghai, Fudan University Shanghai Cancer Center, Shanghai, China; 2 3 Shanghai Medical College, Fudan University, Shanghai, China and University of Michigan, Michigan. Body: Background: Distant metastasis has long been the principal cause of mortality among breast cancer patients. Previous studies found that the age of diagnosis probably played important roles in the prognosis of breast cancer, but few researches focused on its roles on metastatic patients and specific organs involvement. Results: We identified 4932 eligible metastatic breast cancer patients, including 850 younger patients (<50 years), 2,540 middle-aged patients (50-69 years) and 1,542 elder patients (>69 years). Moreover, elder patients were more likely to have bone and lung metastasis, but less likely to have liver metastasis (P<0.
Learning points:Learning points: It is important to buy generic ditropan 5mg on-line gastritis vs heart attack note that research is best learnt by actually conductng a researchIt is important to purchase ditropan 2.5 mg without prescription gastritis lettuce note that research is best learnt by actually conductng a research project generic 5mg ditropan with visa gastritis diet xone, rather than by reading or atending lecturesproject purchase ditropan 2.5 mg amex gastritis diet x1, rather than by reading or atending lectures this handbook is aimed to be used in conjuncton with the user doing an actualThis handbook is aimed to be used in conjuncton with the user doing an actual research project. Learning points:Learning points: this handbook has two examples of research proposals in the appendices. Please refer to the relevant parts of the examples to aid your understanding asPlease refer to the relevant parts of the examples to aid your understanding as you read the various sectons of the handbook. We would like to thank the many researchers who have atended our research workshops over the past 15-20 years. They have helped to shape the contents of this handbook and given valuable feedback. They allowed us to try out many research approaches that enriched our repertoire of research methodology, data analysis and how data can be best presented. A special thanks to the authors of two research proposals who allowed us to reproduce the core summaries of the proposals here as examples. We would also like to thank, in advance, readers of this book who we hope will provide critcal feedback for its improvement. Techniques for data What techniques will best answer the collecton & preobjectve? IntRoDuctIon Write this as one cohesive secton that includes the following components: Identfying & prioritsing problems – write this in terms of why this study is chosen. Literature review – this may be incorporated into the introducton if brief, or writen as a separate secton 3. The researcher uses this secton to crystallize the problem and justfy why it is necessary. A manager or research funding agency wants a clearly outlined argument before funding or approving it. If any of the following is true for your problem, then you don’t need to research it 1. Manager’s most urgent concern that needs research Learning points:Learning points: eliminate non-research problems before prioritzing research problems. This may not refect the most important problems in the community; in additon, difcultes arise when a group of persons try to select a research project. This method allows every member in a group or organisaton to have an equal say in decision making. Spend 10 minutes in silence thinking of important problems that you have at work or in the community. The chairman then lists all the problems on a fip-chart in round-robin fashion untl all problems are exhausted (if tme is a limitaton allow each member to list only his/her two most important problems). Duplicaton establish that the answer is not already available by some other study. Applicability Will the potental soluton be efectve for solving the problem under ideal conditons? If the scope of the study is limited to only a few areas, justfy and give reasons why (see no. Learning points:Learning points: the problem analysis chart will guide the directon of the study. Draw a centre bubble that contains the problem stated in a negatve manner (the primary bubble). In practcal terms the problem analysis is best done by a brainstorming session using a fip-chart. Secondary bubbles are then drawn for key factors contributng to the problem and so on. An evaluaton of the glucose-6 phosphate dehydrogenase screening procedure in selected hospitals in Perak. The argument for why we should conduct this research or why the research problem is important should be clearly outlined. The relevant studies identfed during the literature review are also incorporated into the problem statement. Conclude your introducton with a fnal paragraph clearly statng the importance of the problem and what we aim to do. This is a search for published / unpublished work and experts in the area to guide you in planning your study. The literature review should be incorporated into the introducton, methods and discussion. It is important to have a good and systematc method to keep useful artcles and reference them in the text. Reference them according to one of two internatonal standard formats Vancouver or Harvard styles. Cite artcles used in the text of your proposal/report/publicaton, according to the format selected. Statstcal guidelines for contributors to medical journals Br Med J 1983; 286:1489-1493. Statstcal guidelines for contributors to medical journals Br Med J; 286:1489-1493. Learning points:Learning points: this will help you identfy many things, such as:This will help you identfy many things, such as: •• defnitons of terms. The following table shows some Internet search sites: Name Site Resource Google Scholar htp://scholar. Up-to-date, accurate Medicine gov/database/ informaton about Cochrane Collaboraton htp:// Clearing House gov/ – a public resource for evidence-based guidelines the Community htp://
Cheap 5mg ditropan otc. What is Gastritis?.
Dissemination of Trial Results the sponsor discount ditropan 5 mg visa gastritis causas, investigator and institution have an ethical responsibility to cheap 5mg ditropan with amex gastritis diet and exercise make reasonable efforts to order ditropan 5mg overnight delivery gastritis diet 8 month publicly disseminate the results of clinical research in a timely manner cheap 5mg ditropan mastercard gastritis symptoms yahoo answers. However, it has to be accepted that negative research results are less often submitted and accepted for publication in international medical journals. Proper dissemination of the trial be paid to trials that may include results is, in the first instance, an vulnerable subjects. Some further general details document(s) adequately addresses relevant ethical concerns and are provided on the following pages. Payments to a subject should be prorated and not wholly contingent on completion of the trial by the subject. However, the interpretation and implementation of those and other guidelines are highly dependent on local laws and guidance. A balanced ethics review approach starts with assessment Approval Time of the risk of harm and potential benefits associated with the research (see illustration). An expedited review is by definition completed more rapidly than a full board review, since the type of review the concept of minimal risk provides selected is related to the anticipated risks of harm for the the foundation for a balanced review, and participants. Parts of the magnitude of harm or discomfort text are included as they are related to anticipated in the research are not greater clinical trials on drugs and devices. However, it required to be included in the does specify that each protocol must be continuing progress report. A scientific review judges the who completed the trial; number of importance of the research question and withdrawals from trial to date, due validity of the methodology; this can only to (a) withdrawal of consent, (b) be assessed by those familiar with the loss to follow-up, (c) death; total disciplines and methods of the proposed study withdrawals; number of treatment failures to date, due to (a) research. If yes, give trials overseen by regulatory authorities details; do you plan to increase the planned recruitment of participants will have already been subject to scientific into the study? Any protocol raising many minor concerns or a few major concerns should either be Serious breaches of the protocol or Good Clinical Practice: Have any rejected or subject to revision and serious breaches of the protocol or subsequently re-assessed. Are there any ethical issues on Such trials could also put participants at which further advice is required? The primary goal of continuing ethics review is to ensure continued ethical acceptability of the research. As with initial review, continuing ethics review should be based on a proportionate approach. The renewal must describe current enrolment, ongoing enrolment, adverse events, withdrawals, progress of the trial, and any amendments/changes (see text box on previous page). The review of the suitability of a clinical trial design includes many aspects, and they should be evaluated as an amalgam rather that in isolation, as elaborated in a special section of this Chapter. Major Amendments: Major amendments are defined as any changes that affect the safety or physical or mental integrity of the participants for the conduct or management of the trial. Examples of major amendments are changes in the purpose or design of a trial, substantive changes in procedures used, changes to the trial population such as estimated numbers, age range, inclusion/exclusion criteria, a change of the principal investigator, and changes to trial documentation, such as participant information sheets or consent forms. Minor Amendments: Minor amendments are defined as any changes that do not involve a more than minimum risk for participants or the conduct of the trial. Examples of minor amendments are correcting typographical errors, minor clarifications of the protocol, etc. A substantial amendment is defined as an amendment to the terms of the application, to the protocol, or to any other supporting documentation likely to affect the trial to a significant degree, i. The concept of minimal risk also provides the foundation for a proportionate review of a protocol amendment, i. Researchers must familiarise themselves with the requirements of the sponsor to ensure that the appropriate review is conducted. Important medical events that may not result in death, but are life threatening or require hospitalisation, may be considered serious adverse test article experiences when, based upon medical judgment, they may jeopardise the participant and may require medical or surgical intervention to prevent one of the outcomes listed in this definition. This is in fact a current trend that may become the general practice in the near future – i. Unanticipated Problems A participant protection issue that is often ignored is how investigators identify and manage problems that develop during the course of a trial that are unexpected, related to the research and involve risks to the participants. Having knowledge of unanticipated adverse events or other problems can change the risk-benefit balance in a trial. For example, rather than reporting known and expected adverse events, unexpected adverse events that are related to the research and involve increased risks should be reported. In addition, other types of unanticipated problems can occur, such as test tube mislabeling, breaches in confidentiality, or administration of the wrong dose, even if it results in no harm to participants. Complaints While trials are designed to take into account the interests as well as the safety of research participants, sometimes participants become dissatisfied and wish to file a complaint. When non-compliance is detected, it should be evaluated and appropriate actions should be taken to prevent occurrences of non-compliance to ensure that research participants are protected. Under some laws, serious or continuing non-compliance must be reported to regulatory authorities. Such suspensions should take into account a review of all scientific information as well as the safety and welfare of the enrolled trial participants. Several factors can also influence the decision to prematurely terminate an ongoing clinical trial, including ethical concerns, alterations in standard clinical practise, or reaching a positive or negative statistical end point earlier than anticipated. Termination can also be for financial reasons, such as change of the sponsor’s lead compound priority or diminishing financial resources, including bankruptcy. An individual study site can also terminate its involvement in a trial, owing to factors such as poor recruitment rate, change among site staff, change in research interest, or maybe financial or contractual issues. Compliance with this standard provides public assurance that the rights, safety and well-being of trial participants are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible. The data are intended for use as an important body of evidence when a test article new medicinal product is reviewed by a government regulatory authority.
As a result order 2.5mg ditropan fast delivery gastritis diet yogurt, the sample size required for achievingan 80% power is given by −1 r c 2 (pij − pi·p·j) 9 purchase 2.5 mg ditropan mastercard gastritis help. In a multi-center trial 2.5mg ditropan fast delivery chronic gastritis/lymphoid hyperplasia, it is a concern whether the sample size within each center (or stratum) is sufficient for providingan accurate and reliable assessment of the treatment effect (and consequently for achievingthe desired power) when there is significant treatment-bycenter interaction buy discount ditropan 5mg gastritis symptoms hunger. In practice, a typical approach is to pool data across all centers for an overall assessment of the treatment effect. However, how to control for center effect has become another issue in data analysis. Tests for Goodness-of-Fit and Contingency Tables that a patient in the hth stratum under the ith treatment has response j. The hypotheses of interest are given by H0 : ph,1j = ph,2j for all h, j versus Ha : ph,1j = ph,2j for some h, j. In order to evaluate the power of this test under the alternative hypothesis, H we assume that nh and nh/n → πh,wheren = h=1 nh. Hence, the sample size required for achievinga desired power of 1 − β at the α level of significance is given by (z + z)2 α/2 β n = 2. Suppose the objective of this trial is to compare the test compound with a placebo in terms of the proportions of patients who experience certain types of adverse events. It is expected that the sample size will be approximately evenly distributed across the four centers. Suppose based on a pilot study, the values of ph,ij’s within each country are estimated as follows: Binary Response center Treatment Total 1 Study Drug 0. When the response variable is categorical, this type of change is called a categorical shift. Let xij be the binary response (xij =0: normalandxij = 1: abnormal) from the ith (i =1. Tests for Goodness-of-Fit and Contingency Tables 2 × 2table: Post-Treatment Pre-Treatment Normal Abnormal Normal n00 n01 n0· Abnormal n10 n11 n1· n·0 n·1 n·· where n n00 = (1 − xi1)(1 − xi2) i=1 n n01 = (1 − xi1)xi2 i=1 n n10 = xi1(1 − xi2) i=1 n n11 = xi1xi2. It is then of interest to test whether there is a categorical shift after treatment. A categorical shift is defined as either a shift from 0 (normal) in pre-treatment to 1 (abnormal) in post-treatment or a shift from 1 (abnormal) in pre-treatment to 0 (normal) in post-treatment. Consider H0 : p1+ = p+1 versus Ha : p1+ = p+1, which is equivalent to H0 : p10 = p01 versus Ha : p10 = p01. Notethatdi’s are independent and identically distributed random variables with mean (p01 − p10)andvariancep01 + p10 − (p − p)2. As a result, by the Central Limit Theorem, the power of 01 10 McNemar’s test can be approximated by √ √ n(p01 − p10) − zα/2 p01 + p10 Φ. In practice, however, it is possible that the outcomes are classified into more than two (multiple) categories. Tests for Goodness-of-Fit and Contingency Tables abnormal (two categories), it is often to classify them into three categories. In practice, the data are usually summarized by the following r × r contingency table: Post-Treatment Pre-Treatment ··· r 1 n11 n12 ··· n1r n1· 2 n21 n22 ··· n2r n2· ··· ··· ··· ··· ··· r nr1 nr2 ··· nrr nr· n·1 n·2 ··· n·r n·· Let pij = P(xk1 = i, xk2 = j), which is the probability that the subject will shift from i pre-treatment to j post-treatment. If there is no treatment effect, one may expect that pij = pji for all 1 ≤ i, j, ≤ r. Hence, it is of interest to test the hypotheses H0 : pij = pij for all i = j versus Ha : pij = pij for some i = j. In order to test the above hypotheses, the test statistic proposed by Stuart and Maxwell is useful (see. Hence, for a given significance level of α, the null hypothesis should be rejected if T >χ2. For a given degrees of freedom (r(r − 1)/2) and a desired power (1 − β), δ can be obtained by solving 2 χr(r−1)/2(χα,r(r−1)/2|δ)=β, (6. Test for Categorical Shift 157 where χ2(·|δ) is the cumulative distribution function of the non-central chia square distribution with degrees freedom a and non-centraliity parameter δ. Then, the sample size needed in order to achieve apowerof1− β is given by −1 (p − p)2 ij ji n = δα,β. At the first visit (pre-treatment), patients’ overnight glucose levels will be measured every 15 minutes. At the second visit, patients will receive the study compound and the overnight glucose levels will be obtained in a similar manner. The investigator would like to select a sample size such that there is an 80% (β =0. Stuart-Maxwell Test A pilot study was conducted to study the treatment effect of a test compound based on the number of monocytes in the blood. Tests for Goodness-of-Fit and Contingency Tables followingcontingency table: Post-Treatment Pre-Treatment below normal above below 11 normal 8 above 6 6 25 From this pilot study, the results suggest that the test compound can increase the number of monocytes in the blood because the upper off diagonal elements are always larger than those in the lower off diagonal. To confirm whether such a trend truly exists, a larger trial is planned to have an 80% (β =0. For this purpose, we need to first estimate δ under the given parameters accordingto (6. As a result, the sample size needed for achievingan 80% power is given by −1 (p − p)2 10. When the response is binary, under the assumption of no period and treatment-by-period interaction (carry-over effect), McNemar’s test can be applied to test for the treatment effect. In some cases, the investigator may be interested in testing the treatment-by-period interaction.