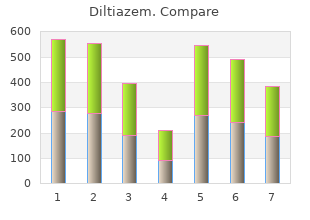
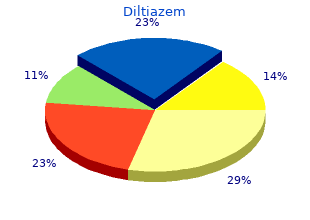
Diltiazem
"Order diltiazem 180 mg overnight delivery, treatment action group."
By: William A. Weiss, MD, PhD
- Professor, Neurology UCSF Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA

https://profiles.ucsf.edu/william.weiss
Digestion of Dietary Proteins Proteins are generally too large to diltiazem 180 mg low cost symptoms you have worms be absorbed by the intestine purchase diltiazem 60mg fast delivery medicine assistance programs. Proteins must order 60mg diltiazem fast delivery treatment goals for ptsd, therefore generic diltiazem 60 mg on line treatment with chemicals or drugs, be hydrolyzed to yield their constituent amino acids, which can be absorbed. Proteolytic enzymes responsible for degrading proteins are produced by three different organs: the stomach, the pancreas, and the small intestine (Figure 3). In contrast to the situation encountered in the newborn infants, in some adult individuals,small amount of intact proteins may be absorbed through the intestinal mucosa. These proteins often cause undesirable immunological responses (formation of antibodies against the foreign protein) and are responsible for the symptoms of food allergies. Digestion of proteins by gastric secretion the digestion of proteins begins in the stomach, which secretes gastric juice—a unique solution containing hydrochloric acid and the proenzyme, pepsinogen. The acid functions instead to kill some bacteria and to denature proteins, thus making them more susceptible to subsequent hydrolysis by proteases. In general, zymogens contain extra amino acids in their sequences, which prevent them from being catalytically active. Figure (3): Digestion of dietary proteins by the proteolytic enzymes of the gastrointestinal tract. Digestion of proteins by pancreatic enzymes On entering the small intestine, large polypeptides produced in the stomach by the action of pepsin are further cleaved to oligopeptides and amino acids by a group of pancreatic proteases 1 Specificity: Each of these enzymes has a different specificity for the amino acid R-groups adjacent to the susceptible peptide bond. For example, trypsin cleaves only when the carbonyl group of the peptide bond is contributed by arginine or lysine. These enzymes, like pepsin described above, are synthesized and secreted as inactive zymogens. Trypsin subsequently converts other trypsinogen molecules to trypsin by cleaving a limited number of specific peptide bonds in the zymogen. Enteropeptidase thus unleashes a cascade of proteolytic activity, because trypsin is the common activator of all the pancreatic zymogens. This results in the abnormal appearance of lipids (called steatorrhea,) and undigested protein in the feces. Digestion of oligopeptides by enzymes of the small intestine the luminal surface of the intestine contains aminopeptidase—an exopeptidase that repeatedly cleaves the N-terminal residue from oligopeptides to produce free amino acids and smaller peptides. Absorption of amino acids and dipeptides Free amino acids are taken into the enterocytes up by a Na+-linked secondary transport system. There, the peptides are hydrolyzed in the cytosol to amino acids before being released into the portal system. Thus, only free amino acids are found in the portal vein after a meal containing protein. These amino acids are either metabolized by the liver or released into the general circulation. Transport of Amino Acids into Cells the concentration of free amino acids in the extracellular fluids is significantly lower than that within the cells of the body. At least seven different transport systems are known that have overlapping specificities for different amino acids. Removal of Nitrogen from Amino Acids Removing the α-amino group is essential for producing energy from any amino acid, and is an obligatory step in the catabolism of all amino acids. Once removed, this nitrogen can be incorporated into other compounds or excreted, with the carbon skeletons being metabolized. Transamination: the funneling of amino groups to glutamate 1-The first step in the catabolism of most amino acids is the transfer of their α-amino group to α ketoglutarate (Figure 4). Glutamate produced by transamination can be oxidatively deaminated (see below), or used as an amino group donor in the synthesis of nonessential amino acids. This transfer of amino groups from one carbon skeleton to another is catalyzed by a family of enzymes called aminotransferases (formerly called transaminases). These enzymes are found in the cytosol and mitochondria of cells throughout the body—especially those of the liver, kidney, intestine, and muscle. All 7 amino acids, with the exception of lysine and threonine, participate in transamination at some point in their catabolism. Figure (4): Aminotransferase reaction using α-ketoglutarate as the amino-group acceptor 1 Substrate specificity of aminotransferases: Each aminotransferase is specific for one or, at most, a few amino group donors. Aminotransferases are named after the specific amino group donor, because the acceptor of the amino group is almost always α-ketoglutarate. The enzyme catalyzes the transfer of the amino group of alanine to α-ketoglutarate, resulting in the formation of pyruvate and glutamate. The presence of elevated plasma levels of aminotransferases indicates damage to cells rich in these enzymes. For example, physical trauma or a disease process can cause cell lysis, resulting in release of intracellular enzymes into the blood. Serial enzyme measurements are often useful in determining the course of liver damage. However, these disorders can usually be distinguished clinically from liver disease. Glutamate dehydrogenase: the oxidative deamination of amino acids In contrast to transamination reactions that transfer amino groups, oxidative deamination by glutamate dehydrogenase results in the liberation of the amino group as free ammonia (Figure 6). They provide α-keto acids that can enter the central pathway of energy metabolism, and ammonia, which is a source of nitrogen in urea synthesis. Glutamate is unique in that it is the only amino acid that undergoes rapid oxidative deamination—a reaction catalyzed by glutamate dehydrogenase.
Feed forward regulation is mediated by anticipatory adjustments in physiological systems based on awareness of a previously experienced or instinctively recognized signal discount 180mg diltiazem amex treatment 3 cm ovarian cyst, preceding any - 183 - Principles of Autonomic Medicine v diltiazem 60 mg lowest price crohns medications 6mp. Feed-forward regulation is more efficient than negative feedback regulation diltiazem 60 mg amex medicine 0636, because it diminishes or eliminates the need for homeostatic adjustments order diltiazem 60mg with visa symptoms 3 dpo. An example is the vagal mediation of the “cephalic phase” of insulin release prior to eating, in anticipation of an increase in blood glucose. Another is the sympathetically mediated hemodynamic changes following “central command” in anticipation of exercise. Concept diagram showing mechanisms of anticipatory and error-controlled regulation. Under ordinary circumstances, levels of the monitored variable are kept within bounds by anticipatory (learned) control. Being reactive, error-controlled regulation is associated with increased variability of the monitored variable. The Figure above depicts in some detail regulation by anticipatory control, which usually is learned and mediated by behavior, and by error control, which is reflexive and mediated by effectors such as components of the autonomic nervous system. Under normal circumstances, in response to anticipation of environmental challenges. The behavior prevents exposure to the environmental challenge from actually altering levels of the regulated variable, in this case core temperature. When anticipatory, learned behaviors are insufficient, and an actual change in the level of core temperature occurs, this evokes reflexive increases in sympathetic noradrenergic and adrenergic outflows, which by cutaneous vasoconstriction and calorigenesis maintain core temperature. The reflexive responses may include certain externally observable behaviors, such as shivering, piloerection, and folding of the arms. Just as insulation in the walls of a house diminishes the requirement of internal adjustment by a furnace in cold weather, many mammals have fur, which creates a layer of motionless air as an insulator above the skin. Other examples of genetically determined insulation based on natural selection in evolution include blubber in whales and closely packed feathers in birds. The barrier to heat loss can be enhanced during more severe cold exposure by reflexive bristling of the hair, mediated by sympathetic nerves; this increases the depth of the layer of motionless air. In addition to these inherited and acquired forms of buffering, countercurrent heat exchange is a relatively common, efficient mechanism present in animals that limits heat loss through the surfaces of the body exposed to an extremely cold environment (polar regions or ice cold water). The preservation of heat is the result of differences in the temperature in closely adjacent arterial and venous blood vessels. Heat from arterial blood warms the cold venous blood returning from the cold surface, diminishing heat loss from the surfaces exposed to the cold. This arrangement occurs in the feet of penguins standing on ice or birds wading in cold water, the paws of the arctic fox, and the tongue of the whale filtering algae from cold water. The heat transfer between the countercurrent flows depends on the difference in the temperatures and does not require energy other than that required to maintain the flows. A somewhat more complex countercurrent system in some - 186 - Principles of Autonomic Medicine v. During inhalation, particularly during panting, moisture evaporation cools the nasal mucosa. A second heat transfer occurs when the cooler venous blood from the nasal mucosa flows into the pterygoid plexus surrounding the carotid rete in a sinus at the base of the brain. Here there is a countercurrent transfer of heat from the warm arterial blood, which flows in the direction opposite to the cooler mucosal venous blood. The cooled arterial blood enters the circle of Willis and is delivered to the brain during exposure to a hot environment. This countercurrent exchange mechanism has been described in detail in sheep and cats and has been reported in a number of other species. Although humans do not have a carotid rete, it has been hypothesized that the diversity in human craniofacial features. A thermostat is a device that compares the temperature that is set with the temperature that is sensed. When the discrepancy is sufficiently large, the thermostat directs changes in activities of the effector, - 187 - Principles of Autonomic Medicine v. The level of the monitored variable, in this case the inside temperature, eventually reaches a stable value. The plateau level may not necessarily be the temperature actually set, because this would depend on factors such as the power of the furnace and efficiency of the insulation. Eventually, the inside temperature is held between what is sensed and what is set. For a given perturbation, the more rapid, sensitive, and powerful the control by negative feedback, the smaller the fluctuations in levels of the monitored variable. When a system regulated by negative feedback is exposed to a fluctuating outside influence, the swings in the levels of the monitored variable are smaller than in the absence of negative feedback. One can conceptualize the existence of many “homeostats,” each with multiple effectors that result in particular neuroendocrine patterns. One can think of a multiplicity of internal homeostatic systems, each with its own “homeostat”—a “barostat” for regulating blood pressure, a “thermostat” for regulating core temperature, a “glucostat” for regulating blood glucose levels, an “osmostat” for regulating serum osmolality, and so forth. In this schema many homeostats use many effectors to keep levels of many monitored variables within bounds via negative feedback loops. A key disadvantage of the homeostat idea is that no evidence has accrued for the existence of physiological homeostatic - 189 - Principles of Autonomic Medicine v. One cannot prove that something doesn’t exist, but at this point it seems best to view homeostats as metaphors, or models for how the regulation happens—i.
Discount diltiazem 180 mg fast delivery. Clarins Skin Care Application method - Face Treatment Oils #enGB.
This means of conceptualizing evolution also helps clarify the possibility that evolution may not always lead to 180 mg diltiazem overnight delivery symptoms adhd a single global maximum of ftness diltiazem 180mg without a prescription medicine daughter lyrics. Your intention is to purchase diltiazem 60mg with mastercard medicine cards climb to generic 60 mg diltiazem fast delivery medicine 801 the highest point possible (maximum ftness) but you cannot see. Regardless of your starting point, the way to get to the highest point would be that every step you take will be up. This will eventually take to you the top of some peak, but you will never know if another mountain exists that is taller. The mechanism by which an organism traverses the ftness landscape is determined by genetic changes and the associated phenotypic changes. A sample evolutionary path has been shown using arrows from the starting point (X) to the ending point (O). Each genetic change also can vary greatly in its efect on an organism’s phenotype. This section will discuss some types of genetic changes that can occur during evolution. Genomic rearrangements are characterized by genetic changes involving a group of nucle otides that change location on the chromosome, are duplicated, or are deleted. It has been estimated that the typical error rate (mutations per base pair per genome replication) varies from 0. In this case, over the course of evolution it would be expected that each site on the genome would have experienced, on average, 27,000 mutations (1. Since specifc codons (groups of three nucleotides) designate specifc amino acids in the translation process, a change in a codon that changes the coded amino acid would result in a potentially large change in the resulting protein. Tese mechanisms typically mediate genomic changes such as deletions, duplications, transpositions, and inversions. Given these two poten tial mechanisms of genomic change, the relative contributions of each in generating genetic variability is an important and unanswered question. While there is a growing body of evidence that genomic 1-6 Evolutionary Tools in Metabolic Engineering rearrangements do contribute to genetic diversity during evolution and can confer an adaptive advan tage, the specifc means by which a genomic rearrangement conveys this advantage may not be entirely clear. It has been observed that cells can adjust the rate at which mutations occur as an adaptive response. The frequency of these recombination-related events is dictated by the activity of recombinases inside the cell, and it has been found that recombinases can be induced under a number of diferent conditions. Regardless of the genetic mechanism behind a phenotypic change, an organism with a large phenotypic advantage is likely to prosper. In evolution ary engineering, the way in which a cellular objective is manifested as a phenotype is of fundamental importance to the design of any project. Tus, it is important to understand what phenotypic traits are benefcial and how these characteristics are brought about during evolution. The most obvious and commonly observed phenotype change is an improvement in cellular growth rate. It is intuitive that evolutionary changes that bring about an increased growth rate would confer an advantage to any cell within a population of growing cells. If a cell grows faster than its neighbors, its progeny will eventually outnumber the slow-growing cells until the slow-growing cells become extinct. This type of phenotype improvement is the most ubiquitous phenotype improvement and represents a dominant selection pressure in microorganisms. A second closely related type of phenotype improvement is an improvement in biomass yield (or metabolic efciency). The contrast between growth rate and biomass efciency was studied explicitly by Helling8 using competing parallel pathways for glutamate synthesis in E. In situations where nutrients are fnite and limited, having improved biomass efciency can be advantageous. As a fnite nutrient becomes depleted, a cell that can satisfy all of its growth requirements using the smallest amount of input would survive longer than one that requires larger amounts of nutrient. The vast major ity of phenotype improvements that are experimentally observed during evolution can be categorized as either growth rate or biomass efciency improvements. Another broad category of evolutionary changes that occur during evolution can be termed either generalist or specialist changes. When applied to phenotype changes, a generalist phenotype improve ment is one where the phenotype change is benefcial to the organism in more than one environment. Evolutionary Engineering of Industrially Important Microbial Phenotypes 1-7 A specialist phenotype improvement is one where the phenotype change is only confers a beneft in one specifc environment and may even be detrimental in other environments. The concept of a specialist phenotype is very closely linked to the genetic concept of Muller’s ratchet. Deleterious mutations over time accumulate within a population due to random drif that causes the irreversible loss of clones that do not house deleterious mutations. Tus, as a population continues to grow in an environment, it remains viable in that environment, but the accumulation of background deleterious mutations becomes sufcient that the strain is efectively crippled for growth in any other environment and is therefore highly specialized and only suited to growth in a single environment. Experimental determination of these types of phenotype improvements is highly dependent upon the diversity of conditions used as phenotype changes may exist but are not refected in the conditions tested and thus remain “silent phenotypes. Cross-feeding is the term given to the situation where a population is com posed of at least two diferent subpopulations. If subpopulation A and subpopulation B existed con currently in the same environment, cross-feeding would be demonstrated by an interconnectedness between the subpopulations where subpopulation A would consume the primary carbon source in the environment and secrete excess carbon as a metabolic by-product. Subpopulation B would then mani fest an adaptation where it would fulfll at least a portion of its metabolic needs by consumption of the by-product secreted by subpopulation A.
One or more specific base triplets (codons) code for an incomplete d-subshell of extranuclear electrons discount 60mg diltiazem medicine knowledge, or which gives each of the 20 amino acids (see genetic code) discount diltiazem 60mg with visa medicine and health. The methionine or manganese diltiazem 60 mg otc symptoms for pneumonia, iron discount diltiazem 180 mg with amex 300 medications for nclex, cobalt, nickel, and copper; the second series is in formylmethionine, or just the formyl group, is removed before the period 5 and comprises those of proton numbers 39 to 47 inclusive, chain is completed. Release factors effect the release of the netium, ruthenium, rhodium, palladium, and silver; the third group complete polypeptide chain and the dissociation of the ribosomal is in period 6 and comprises those of proton numbers 57 to 79 in subunits. The lanthanoids and actinoids are designated inner transition tiates the process of translation of the sequence into an amino acid elements of periods 6 and 7 respectively; their atoms also have in sequence. It is fol translocator or porter 1 a system catalysing a secondary transloca lowed by a state in which more stable intermediates form, prior to tion reaction, i. Consequently, objects cannot be clearly distin processes are of particular value in providing information about the guished through translucent materials. For the different types, see mem transpose 1 to alter the position of; to interchange or place in a dif brane protein. Transposons were first proportion of radiant energy that is transmitted perpendicularly identified as a result of work on transposable elements and also as through a substance or solution; compare transmittance. Each transposon is flanked by repeated sequences, due to transmission factor an alternative name for transmittance. More complex transposons, called composite falling on a body or substance and I the intensity after transmission transposons, may have a central portion having a variety of mark through it. In eukaryotes, transposons constitute much of the repetitive sequences in the genome, with up to a few hundred repeat s = Utr/U0 = exp(–jcl), copies. They are flanked by terminal inverted sequences; the termi where Utr and U0 are the radiant powers of the transmitted and in nal sequences in each strand characteristically represent the com cident radiation, j is the molar napierian absorption coefficient, c plementary bases of the inverted sequence, i. Some transposons move physically from one site to another, while transmitter-gated channel see ion channel. Both mechanisms involve a that objects beyond the transparent material can be clearly distin transposase enzyme; the former also requires a resolvase enzyme. The term can be used in appropriate circumstances to indi transthyretin a thyroid-binding protein occurring in the blood cate materials allowing the passage of other electromagnetic, partic stream, and having an unusual structure. Defects in transthyretin cause various hydrolysis of peptide links, the replacement of one terminal amino forms of amyloidosis and hyperthyroxinemia. The characteristic spin-spin relaxation blood) or across a biological membrane, or of electrons along a se time is normally given the symbol T2. See also active transport, antiport, facilitated diffusion, a purine in place of a pyrimidine, or vice versa. Each member contains six conserved cysteine residues, predicted to form O I three intrachain loops (see P domain). Triacylglycerols are important components of plant oils, ani other a-glucosidases. It is a component of cord factors, which are a mal fats, and animal plasma lipoproteins. The carbohydrate is a compatible solute that acts as a saturated, are solid or semisolid. See also lipoprotein and individual cryoprotectant in many microorganisms, and is used industrially as plant oils and animal fats. They induce formation of a conjugation tube in compatible cell There are various types ranging from those found in microorgan types. That from the yeast Geotrichum group; Tresyl-activated Sepharose can be used in the preparation of candidum is a 544-amino-acid globulin with mainly alpha helix and affinity gels, the sulfonyl groups being readily displaceable by turns; it contains one modified glutamine residue (pyrrolidone car amines, thiols, phenolic groups, or imidazole, which remain bound boxylic acid). It is tricorn protease a protease (1071 amino acids) with trypsin-like not affinity chromatography in the original sense. Aminopepti opment by binding to String (a Cdc25 homologue) and promoting dases with different specificities are usually associated with it. It is extremely toxic to aquatic life and causes se and) chelating a metal ion by means of three donor atoms. The tricaproin a trivial name for trihexanoin, the triacylglycerol contain position of each double bond may be indicated by a locant for its ing three capric (hexanoic) ester residues. It is used in the treatment of manic and schiz acetyl group combines with oxaloacetate to form citrate, which un ophrenic psychoses and as an inhibitor of calmodulin action. One dergoes successive transformations to isocitrate, 2-oxoglutarate, proprietary name: Stelazine (dihydrochloride). In the sheep, S 75% of its amino acids are glutamate or glutamine, arginine or ly sine; the gene is also expressed in epithelia, hoof, and rumen. Tri chohyalin associates in regular arrays with keratin intermediate fil trifluoroacetic acid abbr. Deficiency is associated with glycine; N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; a Good massive excretion of long-chain 3-hydroxycarboxylic acids. The term is commonly used triolein a triacylglycerol in which all the fatty-acyl moieties are in connection with membrane receptor activation and signal trans oleoyl (cis-9-octadecenoyl). It dissociates from the nascent is trapped in a solution of hyamine carbonate for scintillation chain after this is released from the ribosome. It is ex triose phosphate D-glyceraldehyde 3-phosphate, glycerone phos creted in the urine after the consumption of relatively large phate, or a mixture thereof. It is also produced in alfalfa root exu vertible by the enzyme triose-phosphate isomerase.