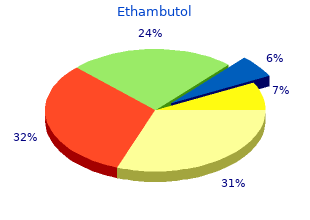
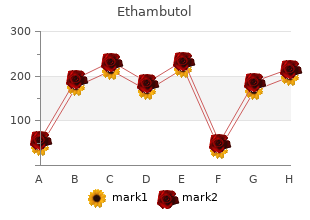
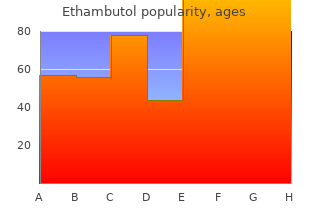
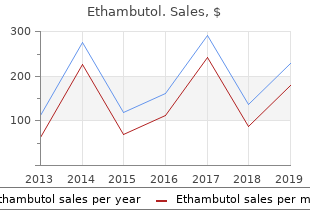
Ethambutol
"Cheap 600mg ethambutol, treatment for dogs eating grapes."
By: Richa Agarwal, MD
- Instructor in the Department of Medicine

https://medicine.duke.edu/faculty/richa-agarwal-md
Impact of infusion set characteristics on the accuracy of care to the home setting order 600 mg ethambutol amex bacteria or virus, include the following patient-controlled morphine administration: a controlled in-vitro factors in the discharge planning process: insur study discount ethambutol 600 mg with amex antibiotics for chest infection. In acute care settings buy cheap ethambutol 800 mg online antimicrobial countertops, no additions gesic pumps at a tertiary care academic medical center order ethambutol 800mg on-line virus 0000. Include physiological, sociological, and psycho acid/dextrose formulations) at least every 24 logical aspects of response to therapy for patients hours. Corkins M, Griggs K, Groh-Wargo S, et al; Task Force on sion-related medication errors, including error Standards for Nutrition Support: Pediatric Hospitalized Patients, American Society for Parenteral and Enteral Nutrition. Reduce the risk of catheter-related bloodstream Society for Parenteral and Enteral Nutrition. Standards not required for adult patients but is recom of practice for nutrition support nurses. Long-term home parenteral nutrition: it light until shortly before the time of administration takes an interdisciplinary approach. Intravenous Medications: A Handbook ensure patient safety, optimal patient outcomes, and for Nurses and Health Professionals. Pharmacopeia/ should include a description of risks, benefits, and National Formulary. Factors deter lung assessment, identification of conditions that mining peripheral vein tolerance to amino acid infusions. Clinical experi an appropriate and patent vascular access device 8,9 ence with three-in-one admixtures administered peripherally. A randomized study of central venous versus rapid transfusion is required, a larger-size cathe peripheral intravenous nutrition in the postoperative period. Neonatal/pediatric patients: umbilical venous life of adult patients treated with long-term parenteral nutrition. Perform patient and blood product identification: and risk of self-harm in patients with central venous catheters. During an independent double check by 2 adults Standard in the presence of the patient (eg, hospital/outpa tient setting: 2 persons trained in the identifica 62. A 1-person verification process may be used administered as quickly as tolerated by the patient 8,13 with automated identification technology (eg, or over 15 to 60 minutes. The use of computerized bar deliver blood or blood components without signifi code-based blood identification systems cant risk of hemolysis of red blood cells. Use only a blood-warming device, with a labeled and do not use if container is not intact or if the indication, when clinically necessary, such as with appearance is not normal (eg, excessive hemolysis, large-volume or rapid transfusions, exchange trans significant color change in blood bag compared to fusions, patients with clinically significant condi administration set, presence of floccular material, tions, and the neonate/pediatric population. The risk cloudy appearance) and return it to the transfusion for clinically important hypothermia is increased 8,13 service. Filter all blood components and follow the manufac with a pressure gauge, totally encase the blood bag, turers directions for filter use. Pressure should not exceed 300 mm harmful particles; standard blood administration Hg. For rapid infusion, a larger-gauge catheter may sets include a 170 to 260-micron filter. Leukoctye reduction filtration is generally pre fusion, after the transfusion, and as needed ferred prestorage or shortly after blood collec depending on patient condition. Initiate the transfusion slowly at approximately 2 cient method and has been associated with dra mL per minute for the first 15 minutes, and matic hypotension in some patients. Stop the transfusion immediately if signs and (V) symptoms of a transfusion reaction are present; 4. Monitor patients for transfusion reactions for at after the completion of each unit or every 4 hours. If least 4 to 6 hours to detect febrile or pulmonary more than 1 unit can be infused in 4 hours, the reactions associated with the transfusion; for transfusion set can be used for a 4-hour period (see patients not under direct observation after the Standard 42, Administration Set Change. Administer and complete each unit of blood or signs and symptoms of a delayed transfusion 7,8,12,18 blood component within 4 hours. Ensure safe transfusion practice if transfusing in an cells or whole blood into smaller aliquots when out-of-hospital setting including the following: doc slower infusion of a unit is required, such as with umentation showing no identified adverse events S136 Copyright © 2016 Infusion Nurses Society Journal of Infusion Nursing during previous transfusions; immediate access to 13. Computerized bar code patient identification and calling for medical assis based identification systems and near-miss transfusion episodes tance if needed; ability to transport blood product in and transfusion errors. Enhancing ability to appropriately dispose of medical waste; transfusion safety with an innovative bar-code-based tracking and a well-designed patient and caregiver education system. Changes in red blood cell integrity monitor recipient adverse reactions and quality con related to infusion pumps: a comparison of 3 different pump trol incidents related to blood transfusions. Transfusion reaction Participation provides organizations with data that identification and management at the bedside. Identify a list of medications that may be adminis Lippincott Williams & Wilkins; 2014:480-529. Neonatal and pediatric transfusion tered by the registered nurse: medications for mod practice. Issues and challenges moderate sedation, airway issues, allergies, and associated with nurse-administered procedural sedation and anal 2,4,7 significant comorbidities. Registered nurse-administered sedation for gastro and for potential need for emergency resuscitative intestinal endoscopic procedure. Nurse administered procedural sedation and analgesia in the cardiac consciousness due to the types of agents used, the 2,4,7 catheter laboratory: an integrative review. Standards for basic anes procedure, including blood pressure, respiratory rate, thetic monitoring. Clinical 2-4,7,8 practice guidelines for evidence-based management of sedoanal other responsibilities during the procedure.
Sexual activity should be avoided with new partners until the warts are gone or removed purchase ethambutol 400mg without a prescription antimicrobial vinegar. This vaccine can prevent most cases of side effects; treatment response and therapy-associated side genital warts in persons who have not yet been exposed effects should be evaluated throughout the course of therapy ethambutol 400mg free shipping bacteria 3 types smear. Persistent hypopigmentation or hyperpigmentation Management of Sex Partners can occur with ablative modalities (e buy ethambutol 400 mg antibiotics for uti at walmart. Special Considerations Cervical Cancer Pregnancy Podofilox (podophyllotoxin) safe 600mg ethambutol bacteria zip line girl, podophyllin, and sinecatechins Screening Recommendations should not be used during pregnancy. Imiquimod appears Recommendations for cervical cancer screening in the United to pose low risk but should be avoided until more data are States are based on systematic evidence reviews and are largely available. Anogenital warts can proliferate and become friable consistent across the major medical organizations, including during pregnancy. Instead, Pap testing is recommended every 3 years Pregnant women with anogenital warts should be counseled from ages 21–29 years. Squamous cell carcinomas arising in or be provided with general recommendations regarding when resembling anogenital warts might occur more frequently to schedule follow-up visits and the importance of cervical among immunosuppressed persons, therefore requiring biopsy cancer screening. Women with abnormal screening tests should for confirmation of diagnosis for suspicious cases (786–788. The cytology can differentiate cells from blood and mucus; importance and frequency of Pap testing or co-testing (Pap conventional Pap test might not. However, in most instances (even in 1) cervical cancer screening in conjunction with a Pap test, the presence of some severe infections), Pap tests will be 2) triage of abnormal cervical cytology results, and 3) follow-up reported as satisfactory for evaluation, and reliable final after treatment of cervical precancers. These tests are only reports can be produced without the need to repeat the approved for use with cervical specimens, not oral or anal Pap test after treatment is received. Women should be counseled on the risks, If the results of the Pap test are abnormal, follow-up care uncertainties, and benefits of screening (126,802. If clinic resources do not allow for follow-up of women with Multiple forms of communication (e. Recommendations and Reports All women should start getting regular Pap tests at age this population. Appropriate follow-up is essential to ensure prevention-and-treatment-guidelines/0) (247. Medications that might cause liver damage or are metabolized by the liver Hepatitis A, caused by infection with the hepatitis A virus should be used with caution among persons with hepatitis A. However, up to 10% of patients experience are prepared from formalin-inactivated, cell-culture–derived a relapse of symptoms during the 6 months after acute illness. A study in persons who are Alaska however, efforts to promote good personal hygiene have not Natives demonstrated that seropositivity for hepatitis A persists been successful in interrupting outbreaks of hepatitis A. Sustained protection and the need for several weeks after onset of symptoms, bloodborne for booster dosing will continue to be assessed (825,826. Transmission by A combined hepatitis A and hepatitis B vaccine (Twinrix) saliva has not been demonstrated. Among adults with identified schedule, the vaccine has equivalent immunogenicity to that risk factors, most cases occurred among sexual and household of the monovalent vaccines. The incubation period from time of exposure indicated because most persons respond to the vaccine. The two available monovalent hepatitis B vaccines among infants and adolescents (4,823,837. In contrast, vaccination coverage among most Serologic marker high-risk adult populations aged ≥30 years (e. The series does not need to be restarted in persons ≥18 years, Twinrix (GlaxoSmithKline Biologicals, after a missed dose. Periodic testing to determine and 6 months; 0, 1, and 4 months; and 0, 2, and 4 months. Pain at the injection site and low-grade When scheduled to receive the second dose, adolescents aged fever are reported by a minority of recipients. For children 16–19 years should be switched to a 3-dose series, with doses and adolescents, a causal association exists between receipt two and three consisting of the pediatric formulation (5 µg) of hepatitis B vaccination and anaphylaxis: for each administered on an appropriate schedule. If the vaccine series is interrupted after the first or known anaphylactic reaction to any vaccine component. Recommended doses of currently licensed formulations of adolescent and adult hepatitis B vaccine have been demonstrated. If hepatitis B § Pediatric formulation administered on a 3-dose schedule; higher doses might be more immunogenic, vaccine is unavailable at a particular facility, but no specific recommendations have been made. Exposed Postvaccination serologic testing for immunity is not persons who are known to have responded to vaccination are necessary after routine vaccination of adolescents or adults. Persons who have written documentation subsequent clinical management depends on knowledge of of a complete hepatitis B vaccine series who did not receive their immune status (e. These persons should be managed according to guidelines exposure to blood or body fluids. Guidelines for management of occupational exposures have been published separately and of chronic hepatitis B infection. Exposed persons who are not fully vaccinated should using an age-appropriate vaccine dose and schedule. Diagnostic and water, sharing eating utensils or drinking glasses, or treatment recommendations for all enteric infections are casual contact. Persons who present with symptoms of acute proctitis should be examined by anoscopy. A Gram-stained smear of any anorectal exudate from anoscopic or anal examination Proctitis, Proctocolitis, and Enteritis should be examined for polymorphonuclear leukocytes. Recommendations and Reports persons with anorectal exudate detected on examination or Allergy, Intolerance, and Adverse polymorphonuclear leukocytes detected on a Gram-stained Reactions smear of anorectal exudate or secretions; such therapy also should be initiated when anoscopy or Gram stain is unavailable Allergic reactions with third-generation cephalosporins and the clinical presentation is consistent with acute proctitis (e. Pediculosis pubis is for acute proctitis should be instructed to abstain from usually transmitted by sexual contact (849.
The Centers for Disease Control and Prevention recommends nucleic acid amplifcation testing for the diagnosis of trichomoniasis in symptomatic or high-risk women buy ethambutol 400 mg mastercard antibiotics herpes. Trichomoniasis is treated with oral metronidazole or tinidazole order ethambutol 600mg with amex infection 1 year after surgery, and patients sex partners should be treated as well order ethambutol 400mg overnight delivery infection control course. Infammatory vaginitis may improve with topical clindamycin as well as steroid application ethambutol 800mg with amex infection from pedicure. Noninfectious causes, including atrophic, lives,2 making it the most common gynecologic diagnosis irritant, allergic, and infammatory vaginitis, are less com in primary care. Studies have shown a negative effect on mon and account for 5% to 10% of vaginitis cases. The most common causes of vaginitis are bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis. For the private, noncom Downloaded from the American Family Physician website at Trichomoniasis Low socioeconomic status, multiple sex partners, other bacterial vaginosis are a cheesy, curdy, or sexually transmitted infections, unprotected intercourse, drug use, smoking focculent discharge; itching; vulvar or vaginal infammation or redness; and lack Atrophic or Menopause, lactation, oophorectomy, radiation therapy, of odor. Patients should be instructed to insert the swab at least one inch into the vagina. Three out of four criteria are required to make the diagnosis, with sensitivity ranging from 70% to 97% and specifcity from 90% to 94%, compared with Gram stain. Routine testing in asymptomatic women and retest recurrent (four or more episodes in one year) or severe ing (test of cure) are not recommended because these bac infections, or infections that occur in a patient who is teria can be part of normal fora. If nonalbicans infection is present, consider first-line ther apy with seven to 14 days of a nonfuconazole azole agent. If infection recurs, prescribe 600 mg of boric acid in a gelatin capsule intravaginally once daily for two weeks. Boric acid may also be used with initial induction therapy followed by monthly maintenance therapy for recurrent albicans infection per the Society of Obstetricians and Gynaecologists of Canada recommendations. These tests have Trichomoniasis is a sexually similar sensitivity and specifcity transmitted infection that to vaginal samples. It can inal wet-mount preparation is promptly There is no cause of vagini be diagnosed when motile, fag examined, motile trichomonads with fa this identifed in up to 30% of ellated protozoa are observed on gella slightly larger than a leukocyte may women. Infammatory endocervical, vaginal, or urine specimens, or on liquid vaginitis is associated with low estrogen levels, such as in based Pap test samples. Physicians should Over-the-counter intravaginal agents explain potential adverse effects with each Clotrimazole 1% cream, 5 g intravaginally daily for seven to 14 days regimen, including a possible disulfram-like Clotrimazole 2% cream, 5 g intravaginally daily for three days reaction with alcohol consumption or gastro Miconazole 2% cream, 5 g intravaginally daily for seven days intestinal symptoms in persons taking oral Miconazole 4% cream, 5 g intravaginally daily for three days metronidazole, or possible weakening of latex Miconazole 100-mg vaginal suppository, one suppository daily for seven days condoms with the use of topical therapies con 9 Miconazole 200-mg vaginal suppository, one suppository daily for taining oil-based preparations. Food and Drug Administration Miconazole 1,200-mg vaginal suppository, one suppository for one day recently approved a single-dose oral therapy for Tioconazole 6. In the past, treatment for bacterial vaginosis during pregnancy was in a single 150-mg dose. In nonpregnant patients, there is no defnitive in preterm labor with treatment of bacterial vaginosis, par advantage of one treatment over another in terms of clin ticularly in early pregnancy (before 20 weeks gestation),46 ical or mycologic cure, with all treatment options having a more recent meta-analysis of 21 studies found that antibi about an 80% cure rate. Oral fuconazole offers the advantage preterm labor before 37 weeks gestation with treatment; of one-dose convenience without messy creams or sup therefore, further investigation may provide more infor positories. Oral medications may cause systemic adverse mation about the role of abnormal bacterial fora and its effects, particularly gastrointestinal effects and toxic treatment in pregnancy. An rial vaginosis is generally recommended for symptomatic additional factor to consider is that topical azole creams relief, and adverse effects of metronidazole in pregnancy and suppositories may be oil-based and can weaken latex have not been demonstrated. There are several topical azole preparations and shown that, regardless of whether they have a history of regimens available, as well as oral fuconazole (Difucan) vulvovaginal candidiasis, women are not able to accurately 326 American Family Physician Ofce-based or laboratory testing should be clotrimazole, miconazole, and keto used with the history and physical examination fndings conazole) are more effective in erad to make the diagnosis. In a small study, topical Do not obtain culture for the diagnosis of bacterial vagi C 9 terconazole was also shown to relieve nosis because it represents a polymicrobial infection. A meta-analysis did not demonstrate clear evidence A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert for probiotics in the treatment of can opinion, or case series. Patients with tion at test of cure and lower rates of reinfection at three complicated vulvovaginal candidiasis require more aggres months. To guide treatment, it is helpful to consider also a frst-line treatment for trichomoniasis; however, it is whether a patient has recurrent infections and whether the more expensive. Metronidazole-resistant trichomoniasis, etiology may be a nonalbicans species of Candida. Trichomoniasis has been second dose of fuconazole given three days after the frst associated with adverse pregnancy outcomes, including dose has been shown to achieve signifcant improvement low birth weight and preterm birth. A second dose did not have signifcant effects for tested and considered for treatment. Atrophic vaginitis is treated with for three doses) followed by weekly treatment with 150 hormonal and nonhormonal therapies. Among hormonal mg fuconazole for six months has been shown to achieve therapies, low-dose vaginal estrogen preparations are symptomatic relief at one year for most patients. Systemic estrogen If severe or recurrent vulvovaginal candidiasis does not therapies are also available for patients with vasomotor respond to initial treatment, culture may guide therapy symptoms. Infections with mendations include vaginal lubricants and moisturizers; nonalbicans species are less responsive to fuconazole. The limited value of symptoms duration of treatment and superiority of one agent over the and signs in the diagnosis of vaginal infections.
The serum adalimumab trough levels at steady-state increased roughly proportionally with dose following 20 cheap ethambutol 600 mg mastercard antibiotics for dogs for sale, 40 and 80 mg subcutaneous dosing every other week and every week generic 600 mg ethambutol fast delivery bacteria 5 types. In adult patients with psoriasis trusted 400mg ethambutol antibiotics for sinus infection and alcohol, the mean steady-state trough concentration was 5 μg/ml during adalimumab 40 mg every other week monotherapy treatment cheap 400 mg ethambutol mastercard antibiotics enterococcus. In adult patients with hidradenitis suppurativa, a dose of 160 mg Humira on Week 0 followed by 80 mg on Week 2 achieved serum adalimumab trough concentrations of approximately 7 to 8 μg/ml at Week 2 and Week 4. The mean steady-state trough concentration at Week 12 through Week 36 were approximately 8 to 10 μg/ml during adalimumab 40 mg every week treatment. In patients with Crohns disease, the loading dose of 80 mg Humira on Week 0 followed by 40 mg Humira on Week 2 achieves serum adalimumab trough concentrations of approximately 5. A loading dose of 160 mg Humira on Week 0 followed by 80 mg Humira on Week 2 achieves serum adalimumab trough concentrations of approximately 12 g/ml during the induction period. In adult patients with uveitis, a loading dose of 80 mg adalimumab on Week 0 followed by 40 mg adalimumab every other week starting at Week 1, resulted in mean steady-state concentrations of approximately 8 to 10 g/mL. After adjustment for weight differences, gender and age appeared to have a minimal effect on adalimumab clearance. Hepatic or renal impairment Humira has not been studied in patients with hepatic or renal impairment. An embryo-foetal developmental toxicity/perinatal developmental study has been performed in cynomologous monkeys at 0, 30 and 100 mg/kg (9-17 monkeys/group) and has revealed no evidence of harm to the foetuses due to adalimumab. Adalimumab is a recombinant human monoclonal antibody produced in Chinese Hamster Ovary cells. Humira can be given as monotherapy in case of intolerance to methotrexate or when continued treatment with methotrexate is inappropriate. Humira has been shown to reduce the rate of progression of joint damage as measured by X-ray and to improve physical function, when given in combination with methotrexate. Humira can be given as monotherapy in case 88 of intolerance to methotrexate or when continued treatment with methotrexate is inappropriate (for the efficacy in monotherapy see section 5. Enthesitis-related arthritis Humira is indicated for the treatment of active enthesitis-related arthritis in patients, 6 years of age and older, who have had an inadequate response to, or who are intolerant of, conventional therapy (see section 5. Psoriatic arthritis Humira is indicated for the treatment of active and progressive psoriatic arthritis in adults when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate. Humira has been shown to reduce the rate of progression of peripheral joint damage as measured by X-ray in patients with polyarticular symmetrical subtypes of the disease (see Section 5. Psoriasis Humira is indicated for the treatment of moderate to severe chronic plaque psoriasis in adult patients who are candidates for systemic therapy. Paediatric plaque psoriasis Humira is indicated for the treatment of severe chronic plaque psoriasis in children and adolescents from 4 years of age who have had an inadequate response to or are inappropriate candidates for topical therapy and phototherapies. Crohns disease Humira is indicated for treatment of moderately to severely active Crohns disease, in adult patients who have not responded despite a full and adequate course of therapy with a corticosteroid and/or an immunosuppressant; or who are intolerant to or have medical contraindications for such therapies. Uveitis Humira is indicated for the treatment of non-infectious intermediate, posterior and panuveitis in adult patients who have had an inadequate response to corticosteroids, in patients in need of corticosteroid sparing, or in whom corticosteroid treatment is inappropriate. Paediatric Uveitis Humira is indicated for the treatment of paediatric chronic non-infectious anterior uveitis in patients from 2 years of age who have had an inadequate response to or are intolerant to conventional therapy, or in whom conventional therapy is inappropriate. Ophthalmologists are advised to consult with an appropriate specialist before initiation of treatment with Humira (see section 4. After proper training in injection technique, patients may self-inject with Humira if their physician determines that it is appropriate and with medical follow-up as necessary. Posology Rheumatoid arthritis the recommended dose of Humira for adult patients with rheumatoid arthritis is 40 mg adalimumab administered every other week as a single dose via subcutaneous injection. Glucocorticoids, salicylates, non-steroidal anti-inflammatory drugs, or analgesics can be continued during treatment with Humira. Regarding combination with disease modifying anti-rheumatic drugs other than methotrexate see sections 4. Available data suggest that the clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be reconsidered in a patient not responding within this time period. Humira may be available in other strengths and/or presentations depending on the individual treatment needs. Dose interruption There may be a need for dose interruption, for instance before surgery or if a serious infection occurs. Available data suggest that re-introduction of Humira after discontinuation for 70 days or longer resulted in the same magnitudes of clinical response and similar safety profile as before dose interruption. Available data suggest that the clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be reconsidered in a patient not responding within this time period. Psoriasis the recommended dose of Humira for adult patients is an initial dose of 80 mg administered subcutaneously, followed by 40 mg subcutaneously given every other week starting one week after the initial dose. Continued therapy beyond 16 weeks should be carefully reconsidered in a patient not responding within this time period. Beyond 16 weeks, patients with inadequate response to Humira 40 mg every other week may benefit from an increase in dosage to 40 mg every week or 80 mg every other week. The benefits and risks of continued 40 mg weekly or 80 mg every other week therapy should be carefully reconsidered in a patient with an inadequate response after the increase in dosage (see section 5. If adequate response is achieved with 40 mg every week or 80 mg every other week, the dosage may subsequently be reduced to 40 mg every other week. Humira may be available in other strengths and/or presentations depending on the individual treatment needs.
Buy discount ethambutol 400 mg. Bioexplore - The Bacterial Ribosome - Mechanism for antibiotic resistance.