Avana
"Purchase 50 mg avana with amex, impotence 28 years old."
By: William A. Weiss, MD, PhD
- Professor, Neurology UCSF Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, CA

https://profiles.ucsf.edu/william.weiss
The more severe syndromes can be life-threatening and the 28 drug must be stopped immediately buy generic avana 200mg line erectile dysfunction drugs free trial. Toxic erythema: Acute morbilliform erythematous eruption that may occur due to drugs generic avana 100mg without a prescription erectile dysfunction drugs reviews. Many agents generic 200mg avana free shipping erectile dysfunction at age 26, hematopoietic growth factors order 200mg avana with amex erectile dysfunction among young adults, Spontaneous resolution occurs over 1–2 weeks, cytokines and interferons are suspected of causing followed by desquamation (quoted from Kane et al, widespread vascular inflammation of skin and 22 visceral organs. Drugs such as hydralazine, antithyroid medications, minocycline, and penicillamine are often associated with antinuclear cytoplasmic antibody or peri- antinuclear cytoplasmic antibody– positive vasculitis-like disease. A Henoch-Schönlein purpura syndrome with cutaneous vasculitis and glomerulonephritis may be induced by carbidopa/levodopa. Respiratory reactions the initial lesions provoke a burning feeling or Airway involvement in drug-induced anaphylaxis pain but not itching. Fixed drug eruption: Hyperpigmented plaques of old lesions with superimposed erythema of new active lesions. Acute generalized exanthematous pustulosis: Numerous confluent pustules superimposed on the erythematous edematous confluent plaques 22 (quoted from Kane et al, 2009). Erythema multiforme Polycyclic target lesions with alternating rings of erythema and dusky desquamation on the arm. Steven-Johnson syndrome: Debilitating mucosal involvement with hemorrhagic ulcerations and crusting requiring 22 hospital admission (quoted from Kane et al, 2009). Bullous Pemphigoid: Itchy bullae that occur as autoimmune response to different antigens, mainly in the old age and tend to concentrate in flexural areas (quoted from Elston 27 and Johnston, 2007) ure 9. Physical examination Immune-mediated hepatocellular necrosis has been should include all systems that could possibly described with methyldopa, halothane, allopurinol, account for the clinical presentation. Haemolytic anaemia can manifestations are the most common presentation 17 be caused by penicillin and methyldopa. Whether the rash is urticarial, maculopapular, It was first described in conjunction with purpuric, bullous or eczematous should be 21 anticonvulsants, but later on, it was ascribed to a established. Investigations the reaction usually develops 2 to 8 weeks after Laboratory evaluation: Routine laboratory therapy is started; symptoms can worsen after the evaluation appropriate to the clinical setting might drug is discontinued and symptoms can persist for be useful for the evaluation of a patient with a weeks or even months after the drug has been suspected drug reaction, depending on the history 33 discontinued. Most patients with drug-induced allergic reactions do not have Diagnosis: eosinophilia, and therefore the absence of History and examination eosinophilia clearly does not exclude a drug- 21 History taking: A detailed history is an essential induced allergic cause. Autoantibodies might be first step towards an accurate diagnosis of a drug- helpful in the evaluation of drug induced vasculitis induced reaction. A thorough Diagnosis of anaphylaxis might be made by history is particularly important when patients are detecting an increase in serum total tryptase levels on several drugs. The diagnosis is aided by a above baseline values or in serum mature tryptase detailed knowledge of the reaction-pattern for each (also known as b-tryptase) levels, which peak 0. Medical notes, drug and nursing charts 2 hours after drug administration and then decrease 37 as well as photographs and eye-witness accounts with a half-life of about 2 hours. Additional should be sought in order to confirm the reaction methods for detecting systemic mast cell mediator 17 and the implicated drug(s). Was the patient taking concurrent sufficient evidence that the patient is at significant medications at the time of the reaction? What was risk of having a type I reaction if the drug is the therapeutic management required secondary to administered. Has molecular-weight drugs, validated and reliable skin the patient experienced symptoms similar to the test reagents are only available for penicillin. Does the negative predictive value of penicillin skin testing patient have an underlying condition that favors (with penicilloyl polylysine, penicillin G, and 21 reactions to certain medications? Thus, although a positive in vitro unless the drug possesses intrinsic histamine- test result for penicillin specific IgE can be highly releasing activity . Intradermal testing requires commercially available tests, are needed before its 43 considerable experience in both technique and general acceptance as a diagnostic tool. If the for the diagnosis of drug-induced eruptions and a test is negative, 10-fold increasing concentrations skin biopsy might not definitively exclude 35 are used sequentially until the test is positive or the alternative causes. Intradermal tests require expert interpretation to Drug provocation tests: Challenge with specific differentiate true positive from irritant reactions and drugs may be carried out after other possible to understand the significance of a negative test. In the majority of cases, it is inadvisable concentrations on the patients back for 48 hours to carry out provocation testing if the reaction has under aluminum discs attached to hypoallergenic resulted in a life-threatening reaction. False negatives occur due to poor challenges and with adequate resuscitation facilities 44 skin penetration by large drug molecules or due to a readily available. A sensitivity range of performed for delayed reactions and it is then between 11% and 43%, has been reported reflecting necessary to give a prolonged course of the 42 different populations selected for patch testing. Challenge testing is contraindicated for of cutaneous drug reactions, including certain types of reactions, e. The starting dose for drug challenge will stages of erythema multiforme major/ Stevens- 35 vary depending on the severity of the previous Johnson syndrome, and contact sensitivities. Conditions to consider in the differential 21 A negative reaction indicates that the diagnosis of drug allergy 46 patient is not sensitive at the time of the challenge. B- • Asthma • Psoriasis blockers should be stopped 24 hours before the 44 • Food allergy • Insect bites/stings drug challenge. For example, a Temporary induction of drug tolerance: morbilliform eruption occurring in a child receiving Definition: Induction of drug tolerance procedures amoxicillin for an upper respiratory tract infection modifies a patients response to a drug to might indeed be due to a viral exanthema and not a temporarily allow treatment with it safely. In addition, patients indicated only in situations where an alternate non– with multiple drug allergies might actually have an cross-reacting medication cannot be used.
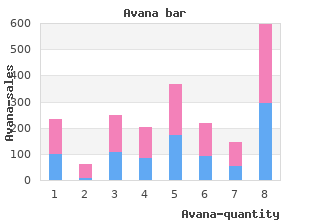
When two different products exist that have dangerously similar labeling/packaging purchase 50 mg avana with amex erectile dysfunction treatment stents, pharmacy 45% 30% 25% seeks an alternate manufacturer for one of the products 100 mg avana with amex impotence from prostate removal. Products with known look-alike drug names are stored separately and not alphabetically generic avana 100 mg on-line erectile dysfunction drugs without side effects, or are 23% 38% 40% clearly differentiated if they remain next to each other discount 100 mg avana free shipping erectile dysfunction pump covered by medicare. Auxiliary warnings, labels with exaggerated fonts, or other label enhancements are used on packages 22% 39% 39% and storage bins of drugs with problematic names, packages, and labels. Special alerts are built into the computer, as necessary, to remind practitioners about 37% 25% 39% problematic or look-alike drug names, packaging, or labeling. Specific items in self assessment - report of current activities: Standardization, Storage, & Distribution Degree of Implementation None Partial Full Access to targeted high-alert medications such as anti-coagulants and oral hypoglycemic drugs, and other problem products, has been safeguarded through constraints (such as drug placement in 47% 30% 22% locked area, removal from fast mover areas where it might be grabbed incorrectly, etc. An automated dispensing system that incorporates robotics and/or bar-code verification systems is 44% 19% 37% used to support the dispensing system in the pharmacy. A mechanism exists to identify the reasons that a prescription has not been picked up after being 33% 26% 41% prepared. Specific items in self assessment - report of current activities: Medication Device Acquisition, Use, and Monitoring Degree of Implementation None Partial Full Patients are instructed on the proper use and maintenance of devices dispensed from the 8% 58% 34% pharmacy . The physical layout of the pharmacy is designed to minimize distractions for pharmacists during the 29% 36% 35% final check in the prescription verification process. When dispensing prescriptions, staff work with one drug product at a time and affix the label to the 7% 35% 58% patients prescription container before working on the next prescription. A device is available and used to hold prescription information near the computer monitor, at eye 21% 16% 61% level, in order to improve visibility when entering orders. Prescriptions are scanned into the computer or received electronically via a hand held device or 86% 3% 10% computer. A magnifying box or lens is in a fixed location and used to facilitate readability of prescriptions and 33% 10% 57% labels. Specific items in self assessment - report of current activities: Staff Competency and Education Degree of Implementation None Partial Full Pharmacy staff is sufficiently trained on the proper use and maintenance of devices dispensed from the 21% 56% 22% pharmacy . Specific items in self assessment - report of current activities: Patient Education Degree of Implementation None Partial Full Adequate time is budgeted by management for 14% 54% 32% patient counseling activities. A suitable private area with minimal distractions is 25% 41% 34% available to provide patient counseling. Clerks fully disclose the intent of the proof of counseling log before asking patients or caregivers 45% 24% 30% to sign the log. Criteria have been established to trigger required counseling and a system is in place to alert the 21% 31% 47% pharmacist of this need. When counseling is provided, the patients drug container is opened in front of the patient/caregiver 28% 56% 16% to verify the appearance of the medication. Patients are informed about the potential for error with drugs that have been known to be problematic 35% 44% 21% and are provided with strategies to help prevent such an occurrence. Management and staff routinely read and use published error experiences from other 37% 38% 25% organizations to proactively target improvements in the medication dispensing process. When switching dosage regimens, administer the first dose of the new regimen on the next scheduled date of the prior regimen. The mean age of the population was 57 years, 49% of the population were women, 85% White, 6% Black, 8% Asians, and 2% other races. The mean age was 56 years (range: 22 to 75 years), 23% were older than 65 years, 52% women, 80% White, 8% Black, 6% Asian; 6% identified as Hispanic ethnicity. The mean age was 57 years (range: 18 to 80 years), 29% were older than 65 years, 49% women, 85% White, 5% Black, 9% Asian; 5% identified as Hispanic ethnicity. Page 5 of 21 Local Injection Site Reactions Injection site reactions occurred in 3. Among the 16,676 patients without diabetes mellitus at baseline, the incidence of new-onset diabetes mellitus during the trial was 8. The mean age was 31 years (range: 13 to 57 years), 49% were women, 90% White, 4% Asian, and 6% other. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed Page 6 of 21 incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies. Patients whose sera tested positive for binding antibodies were further evaluated for neutralizing antibodies; none of the patients tested positive for neutralizing antibodies. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. In animal reproduction studies, there were no effects on pregnancy or neonatal/infant development when monkeys were subcutaneously administered evolocumab from organogenesis through parturition at dose exposures up to 12 times the exposure at the maximum recommended human dose of 420 mg every month. The exposures where immune suppression occurred in infant monkeys were greater than those expected clinically. No assessment for immune suppression was conducted with evolocumab in infant monkeys. Measurable evolocumab serum concentrations were observed in the infant monkeys at birth at comparable levels to maternal serum, indicating that evolocumab, like other IgG antibodies, crosses the placental barrier. Human IgG is present in human milk, but published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
C /C inwomen products were significantly lowerforsubjects with th e C /C T/T vs order avana 50 mg with amex erectile dysfunction mental. A ssociationbetweenlow lactose diets discount 100mg avana with amex erectile dysfunction causes n treatment,lactose intolerance orm alabsorption buy discount avana 100mg line erectile dysfunction and zantac,and clinicalsym ptom s Study C om parison O utcom e C rude odds Ratio (95% C I) Dietary preferences as alifestyle ch oice 32 Black generic avana 50 mg otc impotence 60784,2002 Bad taste ofmilk vs. A ssociationbetweenlow lactose diets,lactose intolerance ormalabsorption,and clinicalsymptoms (continued) Study C om parison O utcom e C rude odds Ratio (95% C I) long-term milk avoidance C a++ intake difference incomparison groups:N R /N R O bjectively detected lactose m alabsorption 11 G oulding,1999 M alabsorbers vs. Gains in osteodensitometric values in prepubertal boys consuming low lactose diet (74% 53 of the recommended daily Ca++ intake) after interventions with dairy foods (1,607 vs. Postmenopausalwomen with outsymptoms C a++ intake difference in comparisongroups:-246/Y 5 DiStefano,2002 L actose intolerance vs. A ssociationbetweenlactose intake and m etabolism and bone density (B D) Study Difference inDaily C a++ C om parison O utcom e Estim ate M eanDifference (95% C I) Intake inC omparisonG roups 16 H onkanen,1996 Positive vs. Evidence table forblinded lactose intolerance treatm entstudies:Q uestion3 and 4 Author, Year, Subject Selection, Study Design, Treatment- Outcome Data Source, Methods Treatment-Active, Study Subject Control, assessment/ Quality of the to Measure Outcomes, Adherence Sponsorship, Characteristics Adherence Results and Study Inclusion/Exclusion Evaluations Country, Length of Evaluations Conclusions Criteria Followup A. An interval of at not reported consumption x 1 of the mean clinical blinded least 72 hours was dose score after both test A statistician. Race/ethnicity: Kluyveromyces aspartame (to was graded as: Intent-to-treat Duration of Inclusion criteria: black 53%, white lactis (Lactaid, simulate the taste 0=none; 1=trivial, analyses: 100% symptom recording: Recurrent abdominal 47%. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup least3 discrete reported h omogeniz ed milk days individualsymptom adequately episodes ofabdominal C ointerventions: (lactose content12 scores was calculated described:no painsevere enough to notreported g)takenfor14 days foreach 14-day study with drawals affectdaily activities for (2 week wash out period and averaged reported 3 month s ormore. Exclusioncriteria: Significantincrease in O rganiccauses of abdominalpainduring abdominalpain. N o sample consisting sample consisting reportingsymptoms concealment: Sponsorsh ip:not experienced furth erinformation oflactose-free milk oflow-lactose milk and meansymptom unclear reported gastrointestinal provided. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup flatulence,abdominal between8 and 10 bloating,abdominal oclock afteran pain,borgorygmiand overnigh tfast nausea were recorded ona questionnaire with a scale rangingfrom 0 (no symptoms)to 10 (verysevere symptoms disturbingnormallife) once every h ourforth e first3 h ours and th en two more times (at4-6 and 7-8 h ours)until8 h ours h ad elapsed since th e testmeal. A sian29% ; lactase preparation breakfastand (moderate),4 Study with drawals K idney Diseases, Inclusioncriteria: H ispanic16% ; obtained from dinner;1 serving (strong),or5 (severe adequately and th e N ational L actose maldigestion black 6% ;wh ite K luyveromyces (28 g)ofa h ard symptoms). Priorto th e study, strawberry flavored, abdominalpain, Exclusioncriteria: 23 womeninth e lactose-h ydrolyz ed diarrh ea,and th e Previously h ad lactose mal- yogurtatlunch. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup h ad significant and 2 inth e lactose th eirregulardiets severity were not intercurrentillnesses, digestiongroup with th e exception significantly different h ad received antibiotic believed th atth e ofth e additional betweenth e 2 th erapy with inth e past2 ingestionofdairy dairy products. Subjective ratings of Study with drawals g/kgofbody weigh t) th e severity of adequately and ifintestinal symptoms (cramps, described:no symptoms occurred. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup symptoms 8 h ours after compared to placebo. Authors concluded aqueous solution Race/ethnicity: lactase-nonpersistent containing15 glactose Asian 69%; black subjects can tolerate was used as th e 8%; Hispanic 15%; two cups of milk per indicatoroflactose white 8% day without malabsorption,h ence appreciable L N P. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup InconsistentG I symptoms (bloating, abdominalpain, flatulence,ordiarrh ea), priorgastrointestinal surgery oroth er significantillnesses, received antibiotic th erapy with inth e past2 month s,orinability to consume aspartame. M eth ods to measure outcomes:Subjects rated symptoms (bloating,borborygmi, abdominalpainor cramps,and subjective impressions ofrectal gas excretion)on4 occasions daily (morning,noon, afternoon,nigh t)during th e baseline and th e 2 testperiods. G ender:women daily (lactose (lactose totaling experienced unclear F innish A ssociation Inclusioncriteria: 90%. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup symptom followup There were no Intent-to-treat duringth e testday. Conclusion(s): A M eth ods to measure marked difference in outcomes:O ntestdays, the fat content of milk afterconsumingmilk, did not affect the subjects noted symptoms of lactose symptoms (flatulence, intolerance. L actose mal- 200 mL fat-free, 200 mL fat-free, Percentage of Allocation R C T,crossover subjects with lactose digesters (n=39) lactose-free milk x lactose-free milk x subjects who concealment: Sponsorsh ip:not maldigestionand 15 Mean age (range): 1 serving (lactose 1 serving (lactose experienced unclear reported lactose digesters. Lactose lemon flavoring and that it contained no abdominal bloating adequately tolerance testwith digesters (n=15) the sweetness and traces of lactose. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup flatulence,were C ointerventions: lactose maldigesters, selected forinth e study notreported ofwh om one-th ird did group. Th e diseases oron same proportion antibiotics one month (64% )ofth e priorto study. K luyveromyces oflactase-treated 0=none;1=trivial; analyses:100% Diabetes and were classified as R ace/eth nicity: lactis (L actaid, milk)atbreakfast 2=mild;3=moderate; followup Digestive and h avinglactose mal wh ite 38% ;A sian Pleasantville,N Y ) daily fora one- 4=strongsymptoms; Study with drawals K idney Diseases, absorptionifth eirbreath 33% ,H ispanic to 1 literofmilk at week period. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup flora to produce G ender:women Duringth e study h ydrogenth rough 56%. W h enth e was tested insevenof C omorbidities:not periods were th e nine subjects wh o reported compared,th ere were were able to absorb C ointerventions: no statistically lactose afterth ey notreported. W h en th erapy with inth e lactose intake is previous two month s;or limited to th e ifth eyindicated th at equivalentof240 ml th eycould notconsume ofmilk orless a day, aspartame. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup N ationalDairy A fricanA mericans. Blinding:double Board In subjects claimed to reported butmostly lactase (L actaid, (to simulate th e 33% (n=10)reported Intent-to-treat cooperationwith th e h ave G I symptoms after female (70% )inth e Pleasantville,N Y ) taste oflactase- symptoms consistent analyses:100% N ationalDairy consumingone cupof eligible population. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup outcomes:Subjects Jersey)or control. Intent-to-treat Denmark two ofth e following: subjects lactase from C onclusion(s): analyses:100% Durationof 1)A nincrease inblood immigrants from K luyveromyces C h ildrenh ad followup symptom recording: glucose duringa lactose K orea,Pakistan,or fragilis (L actoz ym significantly fewer Study with drawals 1 day tolerance test(2 gof Turkey(plus 3 3000 L ,N ovo clinicalsymptoms and adequately lactose perkilogram of native Danes) IndustriA /S, signs with in24 h ours described:no body weigh t); C omorbidities:N o Bagsvaerd, afterconsuming with drawals 2)Diarrh ea, subjects h ad renal Denmark),given lactose-h ydrolyz ed reported borborygmus,and/or orendocrine after8 h ours of milk compared to F unding:non- flatulence duringa disorders or fasting regularmilk. M eth ods to measure outcomes:A t10 times duringth e 24 test D-367 A ppendixTable D8. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup periods,a 0 was recorded inth e scoring ch artto indicate no symptoms and a 1 was recorded ifsymptoms or defecationwas observed by th e ch ildrens parents. Blinding:double reported Inclusioncriteria: R ace/eth nicity: lactose was C onclusion(s): Intent-to-treat Denmark L actose intolerance L atinA merican removed by Ingestionof500 mL analyses:100% Durationof based ona lactose 100% ultrafiltrationand low-lactose milk followup symptom recording: tolerance test(not C omorbidities:not replaced with th e resulted in Study with drawals 8 h ours defined),with no known reported additionofmalto significantly fewer adequately disorders ofth e C ointerventions: dextrose. A fteringestionof250 reported lactose tolerance mL low-lactose milk M eth ods to measure th ere was a tendency outcomes:Subjects to fewersymptoms completed butth e difference was questionnaire notstatistically concerningth e significant. Evidence table forblinded lactose intolerance treatmentstudies:Q uestion4 (continued) A uth or,Y ear, SubjectSelection, Study Design, Treatm ent- O utcom e DataSource,M eth ods Treatm ent-A ctive, Study Subject C ontrol, assessm ent/ Q uality ofth e to M easure O utcom es, A dh erence Sponsorsh ip, C h aracteristics A dh erence R esults and Study Inclusion/Exclusion Evaluations C ountry,L ength of Evaluations C onclusions C riteria F ollowup R ask Pedersen, Data source:11 M eanage:43 500 mL low-lactose 500 mL ordinary N umberofsubjects A llocation 67 1982 symptomaticlactose G ender:women milk (lactose milk (lactose reportingsymptoms concealment: R C T,crossover intolerantDanish adults.
Men in several sub-Saharan African coun- tries avana 100 mg low cost bradford erectile dysfunction diabetes service, such as Namibia cheap 100 mg avana overnight delivery erectile dysfunction at age 28, Botswana generic 50 mg avana with mastercard erectile dysfunction treatment scams, and Sierra Leone order 200mg avana visa erectile dysfunction causes smoking, are in stage 2 (adult prevalence > 20%), as are men in all countries in North Africa [4]. The prevalence of cigarette smoking among adult men in North Africa ranges from 28% in Morocco to 53% in Tunisia (stage 2 or 3), not counting the use of water pipes (shisha and hookah) [4]. In Egypt, 38% of adult men smoke cigarettes, products such as bidis, although indefnitely, by religious and so- and per capita consumption has other tobacco products are also dis- cial norms that discourage female nearly doubled over the past 20 cussed. Most sub-Saharan for men and women in their severity cability of the stages of the epidem- African countries, with the excep- and timing. Public edu- downslope of the epidemic curve the prevalence of cigarette smok- cation about the harmful effects of and remain especially vulnerable to ing in adult men or women is low industry marketing [12,15]. Per capita and implementation of effective to- the prevalence of cigarette smok- consumption is fat or only begin- bacco control policies also curtail ing in men generally increases ning to increase, and there is no tobacco use. Even within a given from north to south, ranging from history of greater smoking in the region there may be considerable 3% in Suriname and Belize to 30% past. In countries where preparation of betel leaf combined with areca nut and/or cured tobacco. Stage 3 is characterized by a fattening or downturn in smoking prevalence (and usually in per cap- ita consumption), coinciding with a continuing steep increase in smok- ing-attributable deaths. Stage 4 is characterized by a decline in both smoking prevalence and smoking- attributable deaths [11]. Several factors infuence the stage of the epidemic within a given country or region. These include the affordability and availability of cigarettes and the intensity of to- bacco industry marketing efforts. The uptake of smoking among women may be deterred, possibly 84 not necessarily follow this gen- in women (16%) than in men [4]. Male smoking preva- use of smokeless tobacco (snus) is mon among women in the Middle lence exceeds 40% in Cuba, Peru, most common in Sweden but is also East, except in Lebanon [4]. Men and Bolivia and exceeds 30% in seen in other Scandinavian coun- are in stage 1 in Qatar, Oman, Venezuela, Uruguay, and Argentina tries [4]. Brazil is the only country in smoking prevalence in men (63%), Arab Emirates and in stage 3 or South America that may be in stage and Austria has the highest preva- late stage 2 in Turkey (48%) [14], 4 for men, with decreases in per lence in women (45%) [4]. Jordan (42%) [4], and the Syrian capita consumption and smoking Countries in the former Soviet Arab Republic (36%) [4]. Use of prevalence in men (22%) and wom- Union have some of the highest water pipes (shisha, hookah, and en (13%) due to rigorous tobacco male cigarette smoking preva- narghile) is common throughout control efforts [13,16]. Male tries in East and South-East Asia in women than those in most of smoking prevalence exceeds 50% [4]. Per capita cigarette con- land, men are in stage 4 of the epi- lence among men has decreased in sumption is still increasing in many demic, whereas women are gener- Poland and Ukraine [13]. Although countries (China, Bangladesh, Ne- ally transitioning from stage 3 to female prevalence does not gen- pal, Viet Nam, Indonesia, and the stage 4. In all of these countries, erally exceed 25% in these coun- Philippines) where men account for male cigarette smoking prevalence tries, it is increasing rapidly in the most of the smoking [14]. Smoking exceeded 50% in past decades Russian Federation and to a lesser prevalence is decreasing among [16]. A concern, lowest smoking prevalence in men sumption in the Russian Federation based on limited anecdotal infor- (13%) and is the only country in has almost doubled in the past mation, is that smoking prevalence Europe where prevalence is higher 20 years [14]. Cigarette consumption and prices in (A) France (1980–2011), (B) Thailand (1990–2011), and (C) South Africa (1961– 2009). Smoking typically begins at Convention on Tobacco Control a large proportion of the anticipated young ages, and Chinese women have reduced tobacco use in many 450 million deaths from tobacco represent a vast untapped market countries. For example, increases over the next few decades by en- for the tobacco companies. This is exemplifed by the to never start smoking in the frst Bangladesh, but the use of smoke- decrease in per capita cigarette place [28]. The prevalence Other important strategies that countries but increasing or persist- of male tobacco smoking is lower in promote cessation and decrease ing at high levels in many low- and India (24%) and Pakistan (28%) [4], initiation include smoke-free laws, middle-income countries. Effective but the use of smokeless products public service counter-advertising, tobacco control strategies such as (paan, gutkha, and betel quid) ex- and restrictions on or banning of large periodic increases in excise ceeds 30% [13]. Strategies to curtail the way on plain packaging (removal epidemic of all graphic branding from ciga- Education about the harms of to- rette packs). The widespread ap- bacco use and policy interventions plication of a few powerful tobacco 86 References 1. The globalization of tobacco use: Smoking and Health: Report of the tion on Tobacco Control. Estimates of Health Service (Public Health Service Effectiveness of Tax and Price Policies global mortality attributable to smoking in Publication No. Targeting the afford- tobacco/surveillance/survey/gats/en/ Tobacco Control in Low- and Middle- ability of cigarettes: a new benchmark for Income Countries [thesis]. Ray Worldwide, tobacco is used in di- hydrocarbons, tobacco-specifc ni- Summary verse smoked or smokeless prod- trosamines (N-nitroso derivatives ucts, all delivering nicotine to their of nicotine and its metabolites), • Tobacco smoke contains more users. The worldwide epidemic of benzene (a cause of leukaemia), than 7000 chemical compounds, tobacco-caused diseases comes formaldehyde (an irritant and car- of which many are known carcin- largely from widespread smoking cinogen), carbon monoxide and cy- ogens. Components of tobacco of manufactured cigarettes, now anide (asphyxiants), acrolein (an ir- smoke contribute to carcinogen- mostly manufactured and distrib- ritant), and polonium (a radioactive esis through multiple pathways, uted by a small number of multi- carcinogen) [2]. In some ted from the smouldering cigarette • Smokeless tobacco products countries, particularly India and is called sidestream smoke. In the contain more than 3000 chemi- Bangladesh, smokeless tobacco presence of smoking, nonsmokers cals and numerous carcinogens.
Baseline and monthly visual acuity and color discrimination monitoring should be performed (particular attention should be given to individuals on higher doses or with renal impairment) purchase 50mg avana amex erectile dysfunction best pills. Dose Adults: 15–20 mg/kg/day frequently divided (max dose 1 gram per day); usually 500–750 mg per day in 2 divided doses or a single daily dose avana 100 mg on-line erectile dysfunction treatment sydney. Children: 15–20 mg/kg/day usually divided into 2–3 doses (max dose 1 gram per day) effective avana 50 mg erectile dysfunction 42. Adults need 100 mg and children should receive a dose proportionate to their weight order avana 100mg erectile dysfunction treatment pdf. Pharmacokinetics Peak oral absorption is usually reached in 2–3 hours, but delayed absorption is common; peak concentrations should be drawn at 2 hours. Special circumstances use in pregnancy/breastfeeding: Generally avoided during pregnancy due to reports of teratogenicity; little data about use during breastfeeding—an estimated 20% of a usual therapeutic dose is thought be received (dose the infant with vitamin B6 if breastfed). Adverse reactions Gastrointestinal upset and anorexia: Sometimes intolerable (symptoms are moderated by food or taking at bedtime). Endocrine effects: Gynecomastia, hair loss, acne, impotence, menstrual irregularity, and reversible hypothyroidism—treat with thyroid replacement. Renal failure/dialysis: Adjustment in dose based on severity of renal failure—for example, 750 mg every 12 hours for creatinine clearance 20–40 ml/min, 500 mg every 12 hours for creatinine clearance < 20 ml/min. Storage Powder should be kept at room temperature; suspended product should be kept no more than 4 hours at room temperature or no more than 24 hours refrigerated. Special circumstances use in pregnancy/breastfeeding: Little information known regarding use in pregnancy; unknown safety during breastfeeding. Contraindications Carbapenem intolerance; meningitis (use meropenem rather than imipenem). Preparation 50 mg, 100 mg, or 300 mg scored or unscored tablets; 50 mg/5 ml oral suspension in sorbitol; solution for injection 100 mg/ml. Pharmacokinetics Peak serum concentrations are achieved at 1–2 hours after the oral dose. Peak concentrations should be drawn at 1 and 4 hours; if other drug concentrations are being submitted, collect blood for peak serum concentrations 2 hours after a dose (and if desired at 6 hours after a dose in order to calculate half-life). Peak concentration is expected to be 3–5 mcg/ml after daily dose and 9–15 mcg/ml after twice weekly dose. Special circumstances use in pregnancy/breastfeeding: Safe during pregnancy; safe during breastfeeding (both baby and mother should receive pyridoxine supplementation). Up to 20% of the infant therapeutic dose will be passed to the baby in the breast milk. Follow-up liver function testing is determined by baseline concerns and symptoms of hepatotoxicity. Therapeutic drug monitoring is recommended only for patients suspected of having malabsorption or treatment failure. If you (or your child) are taking the liquid suspension—do not put it in the refrigerator. Let your doctor know if you get flushing, sweating, or headaches when eating certain cheeses or fish. Cross-resistance with amikacin and some data suggesting cross-resistance with capreomycin; inhibits protein synthesis. Dose (all once daily) Adults: 15 mg/kg/day in a single daily dose, 5–7 days per week (maximum dose is generally 1 gram, but a large, muscular person could receive more and should have concentrations monitored). Markedly obese individuals should have an adjusted dose due to the decreased distribution of extracellular fluids in adipose tissues. For dosing, use adjusted weight as follows: Ideal body weight + 40% of excess weight Ideal body weight (men): 50 kg plus 2. Preparation Clear colorless solution stable at room temperature; 250 mg/ml in vials of 500 mg or 1 gram; 1 gram in 3 ml vial; or 75 mg/vial for infants. Adult doses should be mixed in at least 100 ml of fluid, and pediatric doses should be mixed to a concentration of at least 5 mg/ml. Pharmacokinetics For intravenous administration, infuse over 60 minutes for adults; 1–2 hours for children; intramuscular absorption is complete within 4 hours and peak concentrations are achieved at 1–2 hours. Obtaining a drug concentration 90–120 minutes after intravenous infusion allows for complete distribution of drug. An additional concentration collected 4 hours later will allow for a half-life to be calculated and peak to be back-extrapolated. Presumed to be safe in severe liver disease; however, use with caution—some patients with severe liver disease may progress rapidly to hepato-renal syndrome. Diuretic use: Coadministration of loop diuretics and aminoglycoside antibiotics carries an increased risk of ototoxicity. Adverse reactions Nephrotoxicity: Appears to be more nephrotoxic than streptomycin. Ototoxicity (hearing loss) and vestibular toxicity: Increased with advanced age and prolonged use; appears to occur slightly more commonly with kanamycin than with streptomycin and about the same frequency as amikacin. Contraindications Pregnancy (congenital deafness seen with streptomycin and kanamycin use in pregnancy); hypersensitivity to aminoglycosides; caution with renal, vestibular, or auditory impairment; patients with intestinal obstructions. Monitoring Monitor renal function by documenting creatinine at least monthly (more frequently if renal or hepatic impairment); document creatinine clearance if there is baseline renal impairment or any concerns; document baseline and monthly audiology exam. Question patient regularly about vestibular complaints and perform serial vestibular exams. Document peak and trough concentrations at baseline if there is any question about renal function. Some experts monitor aminoglycoside concentrations routinely, regardless of renal function. Cross-resistance with other fluoroquinolones, but data suggests greater activity than ciprofloxacin or ofloxacin. Usually at least 750 mg/day is used and the dose can be increased to 1000 mg if tolerated.
Cheap 100mg avana otc. Diabetes Role in Erectile Dysfunction: Cause and Cure ( American Diabetes Association Event).